Haber Process - Definition, Process, Reaction, Diagram, FAQs
What is haber process?
Haber process, is also called as Haber-Bosch process, which is an artificial nitrogen fixation process. The Haber process is basically used mainly for the industrial procedure for the production of ammonia. Or this is called the manufacture of ammonia by haber process. The Haber process gets its name by German chemists Fritz Haber and Carl Bosch, who developed Haber process in the first decade of 20th century.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is haber process?
- Haber Process reaction:
- Haber Process diagram:
- Catalyst used in Haber Process:
- Bosch reaction:
The main aim of the Haber process is to convert the atmospheric nitrogen to ammonia with the help of hydrogen and a metal catalyst under high pressure and temperature conditions.
Haber Process:
During world war II the Haber process provided ammonia for production of explosives to Germany. The Haber process is mainly used to produce fertilizers. But the Haber process is considered a sufficiently efficient procedure to produce ammonia. In 20th century ammonia is being prepared by the use of catalysts at high pressure in laboratories. In the year of 1910 Carl Bosch developed industrial level machinery for the production of ammonia. This is the major development in the field of science.
The Haber process is the best way to illustrate the ideas of chemists to implement it according to the needed conditions to produce good products by taking all considerable measures of factors affecting equilibria. In Habers process for ammonia, Nitrogen is treated with hydrogen to produce ammonia. Here we use high temperature and pressure and also a metal catalyst.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
The materials that are used in the Haber process are as follows:
Air, which helps in supplying the nitrogen.
Natural gas and water which helps in the requirement of energy to produce the reactants and also supplying the hydrogen.
Iron works as a catalyst, which is not used up.
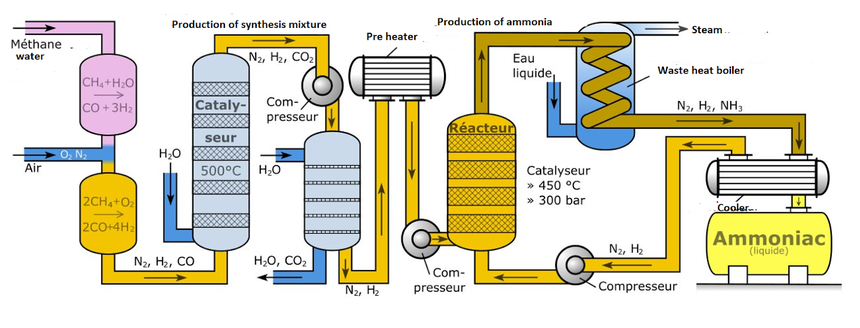
Below showing you the flow chart of ammonia Haber Process.
Haber Bosch Process:
In this Haber -Bosch process, we take hydrogen atom and combine it with nitrogen gas taken from air, and these are in ratio of 1:3 by its volume.
For maintaining the constant equilibrium condition, the gases are passed from the four beds of the catalyst.
Cooling is also maintained in every bed of catalyst.
Unreacted gases are also recycled while passing through different levels.
The catalyst used here is iron maintaining its temperature at 400°-450°C and pressure of about150-200 atm.
The process may include different steps itself such as steam forming, methanation, carbon dioxide removal and shift conversion.
In the last stage of the process the produced ammonia is cooled down in liquid solution, which is further collected and stored.
Haber Process reaction:
The reaction is exothermic as heat is evolved during the production of ammonia. It is also a reversible reaction.
The Haber reaction is as follows:
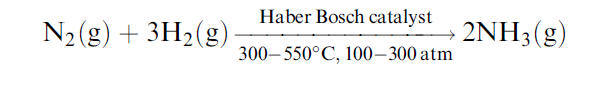
The above-mentioned equation is also called the Haber formula or Haber process equation of manufacturing liquid ammonia under equilibrium conditions.
Haber Process diagram:
Haber Process Temperature:
Haber process temperature can be concluded on the basis of equilibrium considerations, Rate considerations and compromising temperature. Equilibrium Considerations: To maintain the equilibrium in Haber’s process we first need to shift the equilibrium to the right position so as to produce the maximum ammonia out of it. The forward reaction is exothermic in nature.
According to Le Chatelier’s Principle the Haber process will be favoured if the temperature is low. To produce ammonia in Haber process temperature should be low to counteract the effect of position of equilibrium, whereas it is to be noted that temperature will not fall from 400-450℃.Rate Considerations: By lowering the temperature, the reaction becomes slow.
The only thing is to manufacture the ammonia in maximum quantity with very short time the catalyst needs to react in the reactor for the Haber process to complete. The Compromise: By maintaining the temperature 400-450℃, in Haber’s process it can be noted that the production of ammonia is high in the equilibrium mixture in very short period of time.
Related Topics Link, |
Catalyst used in Haber Process:
The reaction used in Haber Process is completed with the help of a catalyst. The catalyst can be used in the reaction under equilibrium considerations, Rate considerations, Separation of ammonia.
Equilibrium Considerations: There is no effect that can be seen by the catalyst in the equilibrium of the Haber Process. The only function of the Haber process catalyst is to increase the speed of the reaction as it will not help in any high production of ammonia.
Rate considerations: If no catalyst is present in the reaction the reaction becomes slow. The requirement is to fasten up the reaction when the gases are in the reactor to achieve the Haber process equilibrium in manufacturing of ammonia.
Separation of ammonia: When the gases react completely in the reactor, we get ammonia under high pressure and temperature. Ammonia is easily liquified under lower temperature, when we lower down the temperature we extract the ammonia and the nitrogen and hydrogen again recycled for the Haber process. Such Process is also called Extraction of ammonia or manufacturing of ammonia by Haber Process.
The catalyst used in manufacturing of ammonia is iron but it is not present in its pure form. It contains some promotor to increase its efficiency such as potassium hydroxide. The iron containing molybdenum can also be used as a catalyst in the manufacturing of ammonia by the Haber process. This gives the answer for how is ammonia manufactured industrially.
Also, students can refer,
- NCERT solutions for Class 11 Chemistry Chapter 6 Thermodynamics
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 6 Thermodynamics
- NCERT notes Class 11 Chemistry Chapter 6 Thermodynamics
Bosch reaction:
The Bosch reaction is the chemical reaction in which the carbon dioxide and hydrogen reacts to produce elemental carbon of graphite form and water. The reaction is named after the German Chemist Carl Bosch. The reaction can be used industrially for manufacturing of hydrogen. The Bosch process helps in producing large amount of hydrogen by only using water and coke.
Bosch Process Equation:
The Bosch process is the most common method of producing hydrogen. As hydrogen is an important compound in every reaction. The Bosch process reaction can be carried out under two major steps as follows:
Step 1: In the first step of Bosch reaction formation of water gas occurs. In this step of Bosh reaction the steam is passed over red hot carbon which produces carbon monoxide and hydrogen gas.
This carbon monoxide and hydrogen gas mixture is called syn gas or water gas. The temperature for such reaction is kept at 1200℃. The Bosch reaction is as follows:
C+H2O→CO+H2
Step 2: In the next step of Bosch reaction production of hydrogen by extracting the hydrogen from carbon monoxide. The water gases will mix up with excess steam and produce carbon oxide and hydrogen gas.
The temperature is kept at 450℃. Iron oxide or chromium oxide is used to speed up the Bosc reaction (catalyst). The reaction is exothermic in nature. The reaction is as follows:
H2+CO+H2O→CO2+2H2
The reaction is Bosch Process Equation.
By separating the carbon dioxide from the water gas mixture by dissolving it in water at 30 atmospheric pressure to form carbonic acid.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Also Read
19 Feb'25 12:52 PM
19 Feb'25 10:40 AM
19 Feb'25 10:36 AM
19 Feb'25 10:35 AM
19 Feb'25 10:16 AM
18 Feb'25 11:45 PM
18 Feb'25 11:43 PM
18 Feb'25 11:40 PM
18 Feb'25 11:38 PM
18 Feb'25 11:36 PM