Haloalkanes Haloarenes - Meaning, Classification, Properties, FAQs
Have you ever wondered how halogen atoms can drastically alter the properties of organic compounds? What happens when halogens like chlorine, bromine, or iodine bond with carbon atoms in alkyl or aromatic structures? You will get these answer by studing this chaoter haloalkanes and haloarenes. The hydrocarbons in which one or more than one hydrogen atom is occupied by the halogens are called haloalkanes. When hydrogen is replaced by halogen in the aromatic hydrocarbon is called haloarenes. They are commercially used in fire extinguishers, refrigerants, and pharmaceuticals.
This Story also Contains
- Haloalkanes
- Monohalogen Derivatives
- Polyhalogen Derivatives
- Halogenation of Alkanes
- Markownikoff’s Rule
- Anti-Markownikoff’s Rule
- Halogen Reactions
- Physical Properties And Chemical Properties Of Haloalkanes
- Haloarenes
- Classification of Halogen derivatives
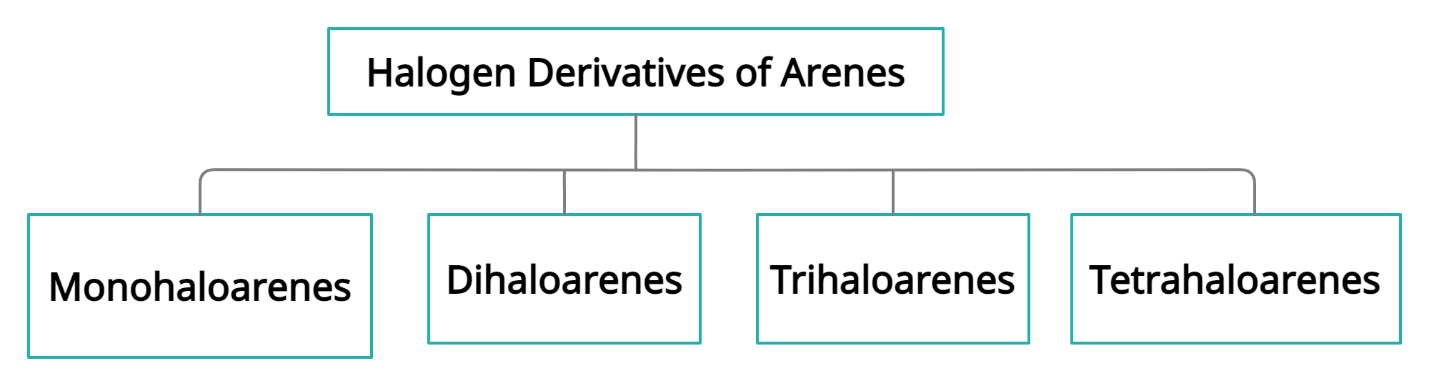
- Monohaloarenes
- Dihaloarenes
- Polyhaloarenes
- Nomenclature of Haloarenes
- Nature of Carbon-Halogen bond in Haloarenes
- Some Solved Examples
.jpg)
In this article, we will cover the topic of Haloalkanes Haloarenes. This topic falls under the broader category of (Haloalkanes And Haloarenes ), which is a crucial chapter in (Class 12 Chemistry). It is not only essential for board exams but also for competitive exams like the JEE Mains Exam ), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more
Haloalkanes
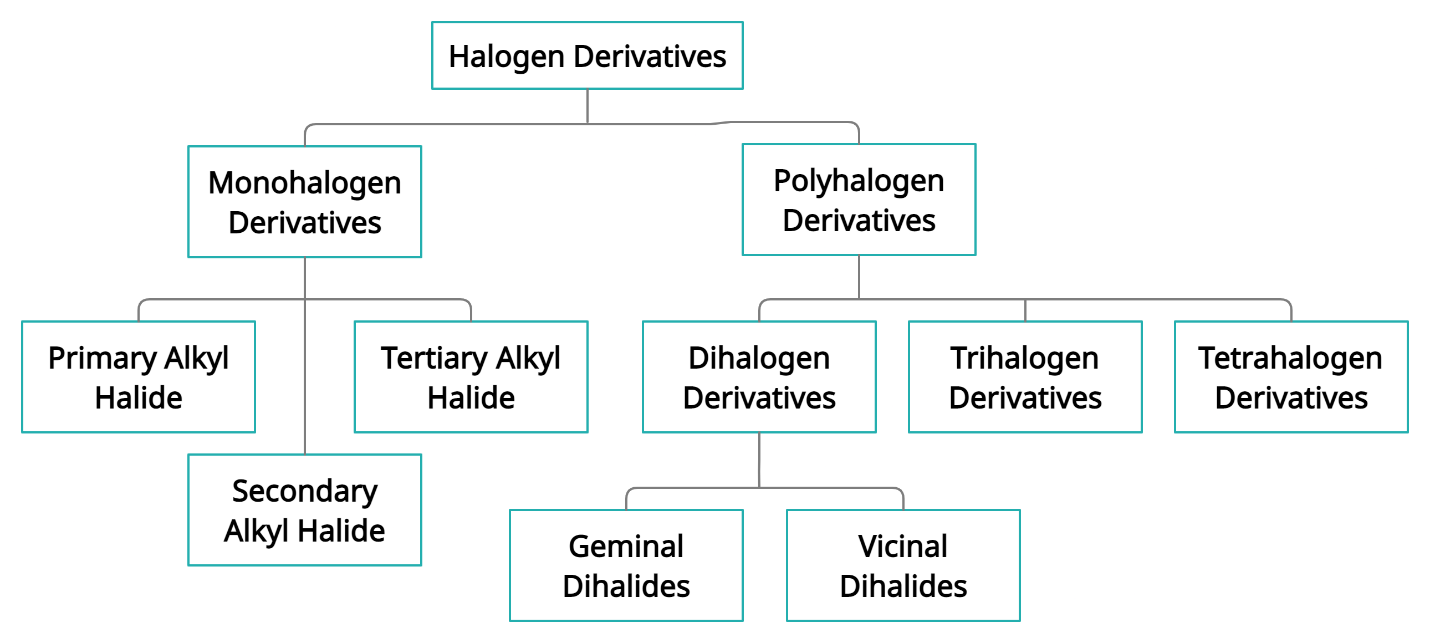
Classification of Haloalkanes
Monohalogen Derivatives
When two or more than two hydrogen atoms of aliphatic hydrocarbon are being replaced by the corresponding number of the halo alkane atom for haloalkanes example chlorine, bromine, or iodine, thus the resulting compounds are known as halogen derivatives of alkanes or haloalkanes. Monohalogen derivatives can be seen in both haloalkanes and Haloarenes. These are represented as R-X, where R is the Alkyl group and X=Br, Cl, I.
The general formula for monohalogen derivatives is$\left(\mathrm{C}_{\mathrm{n}} \mathrm{H}_{2 \mathrm{n}+1} \mathrm{X}\right)$.
$\mathrm{R}-\mathrm{H}+\mathrm{X}_2 \rightarrow \mathrm{R}-\mathrm{X}+\mathrm{HX}$,
where R-H is Aliphatic Hydrocarbon and R-X, is Alkyl Halide.
For haloalkanes examples:
$\mathrm{CH}_3+\mathrm{Cl}_2 \rightarrow \mathrm{CH}_3 \mathrm{Cl}+\mathrm{HCl}$
All the saturated aliphatic hydrocarbons are called alkanes (CnH2n+2). Also, the halogen atoms contain halogen atoms which are attached to the sp3 hybridized carbon atoms.
Primary Alkyl Halide
Alkyl Halides which has a halogen atom bonded with primary carbon atoms are known as primary alkyl halide. The general formula is given by R-CH2-X.
Secondary Alkyl Halide
Alkyl halide which has a halogen atom bonded with a secondary carbon atom is known as secondary alkyl halide. The general formula is given by R-(R')CH-X.
Tertiary Alkyl Halide
Alkyl Halide which has halogen bonded with primary carbon atoms is known as primary alkyl halide. The general formula is given by R-(R')C(R")-X.
Polyhalogen Derivatives
Similarly, when more than one atom of hydrogen of alkanes is substituted by the other corresponding number of halogen atoms the following compound is called as polyhalogen derivative of alkanes. Similarly, Polyhalogen derivatives can be seen in both haloalkanes and haloarenes. Further on the basis of the number of halogens present in molecules are classified as follows as:
-
Dihalogen derivatives: Two hydrogen atoms of alkane which is substituted by the two halogens which form the following compound, and it is called as Dihalogen derivative of alkane. The general formula is given by CnH2nX2. Dihalogen derivatives are further classified into:
-
Vicinal Dihalides: Two halogen atoms that are present on the adjacent carbon atoms. E.g., Ethylene dichloride.
-
Geminal Dihalides: Two halogen atoms that are present on the same carbon atom. E.g., Ethylidene dichloride.
-
Trihalogen derivatives: When three hydrogen of alkane which is substituted by the other three halogen atoms following compound is known as the Trihalogen derivative of the alkane. The general formula is given by CnH2n-1 X3.
-
Tetrahalogen derivatives: When four hydrogen of alkane which is substituted by other four halogen atoms following compound is known as the Tetrahalogen derivative of alkane. The general formula is given by CnH2n-2X4.
Halogenation of Alkanes
Replacement of one or more than hydrogen of alkanes by halogen for haloalkanes example Chlorination, Bromination, and Iodination of alkanes. A halogenation reaction is a chain reaction that proceeds by the formation of free radicals. Also, the halogenation reaction is for both haloalkanes and haloarenes.
This halogenation continues till every hydrogen in alkane is replaced by halogen.
-
Chlorination: When alkanes are heated with chlorine in the presence of UV light or at high temperatures it forms alkyl chlorides. Generally, chlorination is given by
$\mathrm{R}-\mathrm{H}+\mathrm{Cl}_2\xrightarrow{\text { UV light }} \mathrm{R}-\mathrm{Cl}+\mathrm{HCl}$
-
Bromination: When alkanes are heated with bromine in the presence of aluminum tribromide (any. AlBr3) with the corresponding formation of alkyl bromide. Bromination is given by
$\mathrm{R}-\mathrm{H}+\mathrm{Br}_2 \xrightarrow{\text { anhyAlBr }} \mathrm{R}-\mathrm{Br}_2+\mathrm{BHBr}$.
-
Iodination: When alkane is treated with iodine the following product formed is alkyl iodide. However, Hydroiodic acid is more stronger reducing agent which also reduces alkyl iodide back to alkane by making the iodination reaction a reversible reaction. The reaction here needs to be processed out in the presence of oxidizing agent haloalkanes example mercuric acid, Iodic acid, dil. Nitric acid, etc. This oxidizing agent prevents backward reaction by decomposing the hydroiodic acid (HI) formed. The general representation is given by
$\mathrm{R}-\mathrm{H}+\mathrm{I}_2 \rightarrow \mathrm{R}-\mathrm{I}+\mathrm{HI}$
Markownikoff’s Rule
When unsymmetrical haloalkenes are treated with an unsymmetrical reagent which is HX than the negative part of the reagent is (-X) which gets added to the unsaturated carbon of double bond this contains less number of hydrogen. The general formula is given by
$\mathrm{R}-\mathrm{CH}_2-\mathrm{CH}_3+\mathrm{HX} \rightarrow \mathrm{RCHXCH}_3$
Anti-Markownikoff’s Rule
When unsymmetrical haloalkenes are treated with an unsymmetrical reagent which is HX in the presence of peroxide (Na2O2) then the negative part of the reagent is (-X) which gets added to the unsaturated carbon of double bond this contains more number of hydrogen. The general formula is given by
$\mathrm{R}-\mathrm{CH}=\mathrm{CH}_2+\mathrm{HBr} \xrightarrow{\text { Peroxide }} \mathrm{RCH}_2 \mathrm{CH}_2 \mathrm{Br}$
Major Product (1-Bromoalkane)
The most important thing is that the peroxide effect is shown only and only by the HBr meanwhile HCl and HI always is added according to Markownikoff’s rule.
Related Topics Link
Halogen Reactions
Finkelstein Reaction
The Finkelstein reaction is used for the preparation of alkyl iodide. Alkyl bromide/chloride is heated with sodium iodide solution in Acetone(dry) and forms alkyl iodide. NaCl and NaBr are way less soluble in Acetone(dry) and it gets precipitated further these precipitates is removed by the filtration process along the backward reaction is prevented.
$\mathrm{R}-\mathrm{X}+\mathrm{NaI} \xrightarrow{\text { Dry acetone }} \mathrm{RI}+\mathrm{NaX}$
Alkyl halide Sodium Iodide Alkyl Iodide Sodium Halide
Swarts Reaction
Similarly, the alkyl fluorides which can be prepared by the Swarts reaction method by the reaction of Mercurous fluoride, Silver fluoride, Cobalt fluoride, or Antimony fluoride on alkyl chloride/bromide.
$2 \mathrm{R}-\mathrm{X}+\mathrm{Hg}_2 \mathrm{~F}_2 \rightarrow 2 \mathrm{RF}+\mathrm{Hg}_2 \mathrm{X}_2$
Alkyl Halide Mercurous Fluoride Alkyl Fluoride (Fluoroalkane)
Substitution Reaction
When an atom or group of atoms are substituted or replaced from the substrate the number of other atoms or groups is known as substitution reaction.
$R-X+Y^{-} \rightarrow R-Y+X^{-}$
Alkyl Halide Nucleophile Substituted Alkane Halide Ion
Where Y- is a nucleophile like OH-, CN-, NH2-, R-O-, RCOO-, I-, etc.
Elimination Reaction
A reaction in which two atoms are removed from the adjacent carbon atom in a molecule which forms an unsaturated compound which is known as an elimination reaction.
Williamson’s synthesis of Ethers
Alkyl halide is heated with the alkali alkoxide to give respective ether. In this reaction, the halide group undergoes substitution with an alkoxy group (-O-R'). For example, sodium alkoxide can be prepared by the action of sodium metal on alcohol.
R-X+NaOR' $ \xrightarrow{\text { Heat }}$ R-O-R'+NaX
Alkyl Halide Sodium Alkoxide ether
Physical Properties And Chemical Properties Of Haloalkanes
-
The lower elements are gases at room temperature and the higher elements are solids or liquids.
-
The more volatile halogen compound is more sweetest smell it has.
-
The boiling point of the Alkyl halide is always greater than the respective hydrocarbons.
-
Since the polarity of the Carbon-halogen bond along with the dipole-dipole-London force and Van der Waal’s between the molecules of haloalkanes are stronger thus there is an increase in boiling point.
Haloarenes
When one or more than one hydrogen atom of an aromatic hydrocarbon is substituted by the respective number of halogen atoms the major product form from the reaction is called Haloarenes. The general formula for haloarenes is given by Ar-X, where Ar is the aryl group and X is halogen i.e., F, Br, Cl, I. Aromatic hydrocarbons are known as arenes. Also, Haloarenes contain halo alkane atoms attached to sp2 hybridized carbon atom.
Classification of Halogen derivatives
Monohaloarenes
When one hydrogen atom of arene is replaced by one halogen the following compound formed is known as monohaloarenes. Example: Chlorobenzene, Bromobenzene.
Dihaloarenes
When two hydrogen atoms of arene are replaced by two halogens the following compound formed is known as Dihaloarenes. Example: 1,2-Dichlorobenzene, 1,4-Dibromobenzene.
Polyhaloarenes
When two or more hydrogen atoms of arene are replaced by the same number of halogens, the following compound formed is known as Polyhaloarenes. Example: 1,3,5-Tribromobenzene (Trihaloarene), 1,2,3,5-Tetrachlorobenzene (Tetrahaloarene).
Nomenclature of Haloarenes
-
Aryl halides are named by prefixing the 'halo' to the name of the parent aromatic hydrocarbon.
-
For the Dihalogen derivative, the prefixes o-, m-, and p- are used in the common system but in the IUPAC system, numerical prefixes (1, 2) (1, 3) and (1, 4) are respectively used.
-
In case, the parent aromatic hydrocarbon carries a side chain or substituent, then the numbering of carbon atoms begins with the carbon atom attached to the halo atom.
-
However, polyhalogen derivatives do not have common names (Except three groups in symmetrical position, the suffix `sym' is used) but only have IUPAC names in which positions of halogens are indicated by Arabic numerals.
Nature of Carbon-Halogen bond in Haloarenes
In haloarenes, lone pairs of electrons on the halogen atom are in conjugation with the electrons of the benzene ring. In the case of chlorobenzene, a lone pair of electrons from the chlorine atom as well as six electrons from the carbon atoms of the ring are thereby associated with all seven atoms (six carbon atoms and one halogen atom). The delocalization of electrons gives the double bond character to the C—X bond.
Polyhalogen Compounds: Carbon compounds which have more than one halogen atom it is known as polyhalogen compounds. Some of the Haloarenes example: p,p’-Dichlorodiphenyltrichloroethane (DDT); Freons (CCl2F2); Iodoform (CHI3); Tetrachloromethane CCl4; Trichloromethane (CHCl3); Dichloromethane (CH2Cl2).
Also read -
Some Solved Examples
Question 1: Choose the correct option regarding the quantum yield of photosynthesis of.
1) (correct) $\mathrm{HCl}>\mathrm{HBr}$
2) $\mathrm{HCl}<\mathrm{HBr}$
3) $\mathrm{HCl}=\mathrm{HBr}$
4) None of these
Solution:
The quantum yield of photosynthesis is a measure of how efficiently absorbed light produces a specific effect. It is calculated by dividing the rate of a light-dependent process by the rate at which the system absorbs photons. It can also be defined as the number of oxygen molecules produced by one quantum of light, or the moles of carbon dioxide fixed per mole of quanta absorbed.
The quantum yield of photosynthesis for hydrogen chloride (HCl) is higher than that for hydrogen bromide (HBr). Quantum yield is the number of molecules that react or products that form per photon absorbed by a system during a radiation-induced process.
The higher quantum yield for HCl is due to the first step of the secondary process being exothermic, meaning it happens easily and immediately after the primary process. This exothermic step starts a chain reaction, which results in a higher yield per photon absorbed. In contrast, the first reaction of the secondary process for HBr is endothermic, which means it has fewer chances of happening, so there is no chain reaction. This results in a lower yield of HBr per photon absorbed.
Photosynthesis of HBr has a low value of , while photosynthesis of HCl has very high volume of .
Hence, the answer is the option (1).
Question 2: Arrange the following in the correct order of decreasing bond enthalpy
$$
\mathrm{H}_3 \mathrm{C}-\mathrm{F}, \mathrm{H}_3 \mathrm{C}-\mathrm{Cl}, \mathrm{H}_3 \mathrm{C}-\mathrm{Br}, \mathrm{H}_3 \mathrm{C}-\mathrm{I}
$$
1) (correct)$\mathrm{H}_3 \mathrm{C}-\mathrm{F}>\mathrm{H}_3 \mathrm{C}-\mathrm{Cl}>\mathrm{H}_3 \mathrm{C}-\mathrm{Br}>\mathrm{H}_3 \mathrm{C}-\mathrm{I}$
2)$\mathrm{H}_3 \mathrm{C}-\mathrm{Cl}>\mathrm{H}_3 \mathrm{C}-\mathrm{F}>\mathrm{H}_3 \mathrm{C}-\mathrm{Br}>\mathrm{H}_3 \mathrm{C}-\mathrm{I}$
3)$\mathrm{H}_3 \mathrm{C}-\mathrm{I}>\mathrm{H}_3 \mathrm{C}-\mathrm{CBr}>\mathrm{H}_3 \mathrm{C}-\mathrm{Cl}>\mathrm{H}_3 \mathrm{C}-\mathrm{F}$
4)$\mathrm{H}_3 \mathrm{C}-\mathrm{I}>\mathrm{H}_3 \mathrm{C}-\mathrm{CBr}>\mathrm{H}_3 \mathrm{C}-\mathrm{F}>\mathrm{H}_3 \mathrm{C}-\mathrm{Cl}$
Solution:
As we learned
Carbon halogen (C-X) bond length, Bond enthalpy, and dipole moments -
These factors depend on the nature of C-X bonds.
- wherein
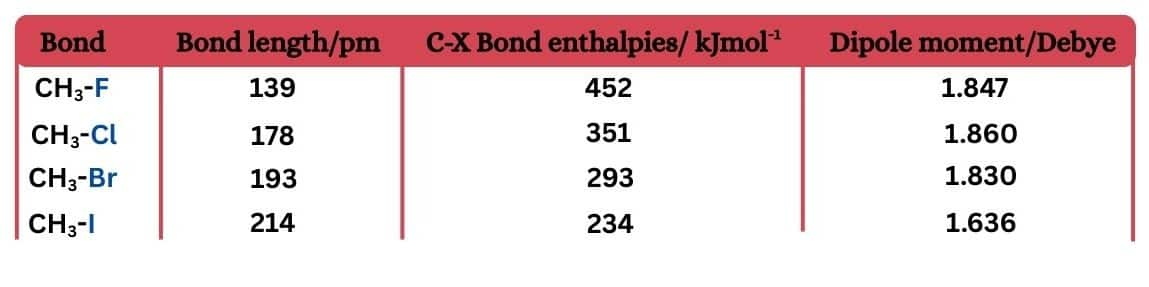
In haloalkanes, the bond strength of the carbon-halogen bond decreases with an increase in bond length, as one moves from fluorine to iodine. So the order will be
$\mathrm{H}_3 \mathrm{C}-\mathrm{F}>\mathrm{H}_3 \mathrm{C}-\mathrm{Cl}>\mathrm{H}_3 \mathrm{C}-\mathrm{Br}>\mathrm{H}_3 \mathrm{C}-\mathrm{I}$
Hence, the answer is option (1).
Question 3: $\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{X}+\mathrm{KCN} \xrightarrow{\text { alcohol }}$
The major product of the above reaction is:
1) (correct)$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{CN}$
2)$\mathrm{CH}_3 \mathrm{CN}$
3)$\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{OH}$
4)$\mathrm{CH}_3 \mathrm{COOH}$
Solution:
As we learnt
Reaction of alkyl halides with KCN -
Alkyl halides, when treated with KCN, form Alkane nitrile, a major product.
$
\mathrm{KC} \equiv \mathrm{~N}+\mathrm{CH}_3 \mathrm{CH}_2 \mathrm{X} \longrightarrow \mathrm{CH}_3 \mathrm{CH}_2-\mathrm{C} \equiv \mathrm{~N}+\mathrm{KX}
$
Hence, the answer is option (1).
Practice More Questions With the Link Given Below
Frequently Asked Questions (FAQs)
Haloalkanes are used in pharmaceuticals, pesticides, solvents, refrigerants, and as intermediates in organic synthesis. They can also be found in certain cleaning products and are used in the production of other chemicals.
Haloalkanes can be synthesized through several methods, including:
- Halogenation of alkanes using halogens in the presence of UV light.
- Reacting alcohols with hydrogen halides (HX) to produce haloalkanes.
- The reaction of alkenes with halogen halides.
Many haloalkanes and haloarenes can be toxic, with potential health hazards including carcinogenic effects, neurological impacts, and skin irritation. Safety measures should be taken when handling these compounds.
Haloarenes is less reactive than haloalkanes due to haloarenes electron pair on halogen atom is in conjugation with pi electron of ring. Since the bond cleavage in the haloarenes is difficult than haloalkanes. Since resonance carbon-halogen in aryl halide because it possesses double bond character. Thus, haloalkanes are more reactive than haloarenes.
Alkyl halides are soluble in water and due to intermolecular forces of the attraction moreover, they are soluble in organic solvents too.
Halogen containing organic elements/compounds which is used as a solvent for non-polar. Alkyl halide is used in insecticides, and it contribute to human health sectors too.
Alkyl halide can be prepare using alcohol by replacing hydroxyl group of alcohol with halogen atom group.