Heterogeneous Mixture Homogeneous Mixture - Definition, Difference, Examples, Reaction, FAQs
Homogeneous mixture and heterogeneous mixture Definition: The mixture in which the composition is uniform throughout the solution or any other substance. Basically, the meaning of the word ‘Homo’ means the same everywhere.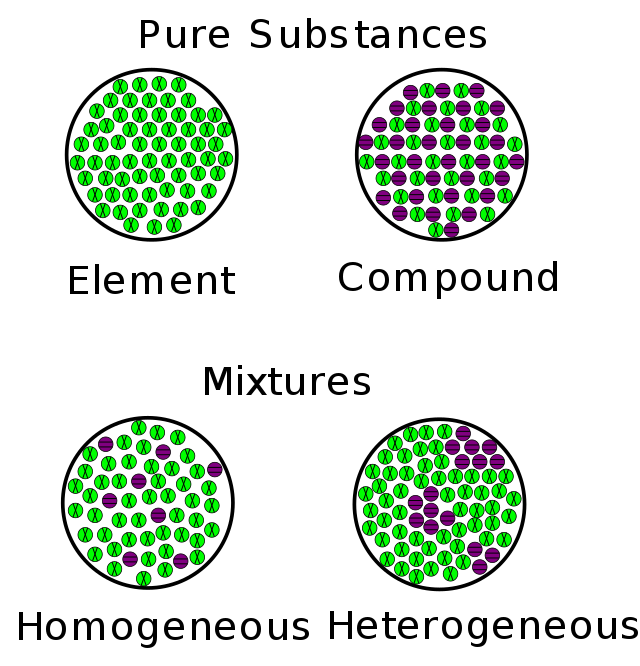
This Story also Contains
- What is a heterogeneous mixture?
- Difference between homogeneous mixture and heterogeneous mixture:
- Various Example of Homogeneous mixture and heterogeneous mixture:
- Homogeneous mixture and heterogeneous mixture Reaction:
Concept or definition of mixture:
Homogeneous meaning: Let us perceive other ideas regarding solid mixture and heterogeneous mixture as an example, if we tend to dissolve salt within the water, the mixture is solid because of the dissolved salt is distributed uniformly or equally throughout the complete brine answer. Usually, it's straightforward to confuse a solid mixture with a pure substance because of they're each uniform. The distinction is that the composition of the substance is often similar homogeneous mixture and heterogeneous mixture, the quantity of salt within the salt water will vary from one sample to a different. All solutions would be thought about solid because the dissolved material is gift within the same quantity throughout the answer.

Example of homogeneous mixture i.e. when we dissolve color in water it is uniformly distributed.
Also read -
What is a heterogeneous mixture?
Heterogeneous Mixture: may be the mixture with non-uniform composition. The composition varies from one region to a different with a minimum of two phases that stay independent from one another, with clearly acknowledgeable properties. If you examine a sample of a homogeneous mixture and a heterogeneous mixture, you'll see the separate elements. In chemistry, the definition of a heterogeneous mixture is somewhat totally different. Here, a consistent mixture is one during which all elements area unit in a very single section, whereas a heterogeneous mixture contains elements in several phases.
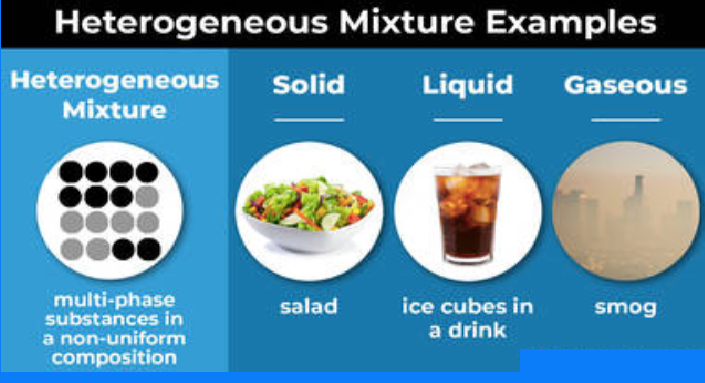
Examples of heterogeneous mixtures in different phases.
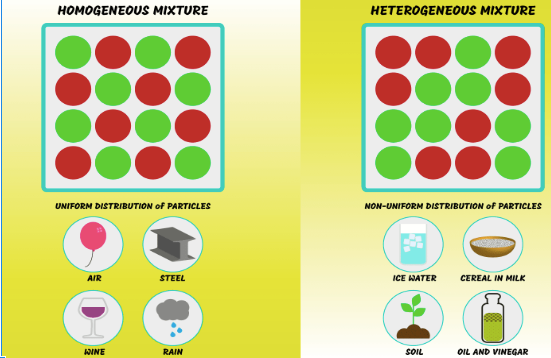
Difference between homogeneous mixture and heterogeneous mixture:
| Homogeneous mixture | Heterogeneous mixture |
| The composition is uniform in the solution. | The composition is non- uniform. |
| to separate homogeneous mixture physically is impossible. | we can separate heterogeneous mixture physically. |
| The word ‘homo’ means the same. | The word ‘hetro’ means different. |
| this mixture has only one phase | This mixture contains two phases in a system. |
| Example: a mixture of alcohol and water. | Example: a mixture of sodium chloride and sand. |
Above table shows the difference between homogeneous and heterogeneous mixture.
Also Read:
Various Example of Homogeneous mixture and heterogeneous mixture:
Homogeneous mixture examples:
- Air
- Sugar water
- Rainwater
- Vodka
- Vinegar
- Dishwashing detergent
- Steel
Heterogeneous mixture examples:
- Cereal in milk
- Vegetable soup
- Pizza
- Blood
- Gravel
- Ice in soda
- Salad dressing
- Mixed nuts
- Bowl of colored candies
- Soil
Homogeneous mixture and heterogeneous mixture Reaction:
Homogeneous reaction:
Homogeneous reactions square measure chemical reactions during which the reactants and merchandise square measure within the same section of matter. There are sections of matter, solid phase, liquid section, and gas section. If the reactants of an undiversified reaction square measure within the gas section, then the merchandise given by that reaction are within the gas section. The most necessary homogenized reactions square measure the reactions between gases and reactions between liquids or substances that square measure dissolved in liquids.
Related Topics Link
- Mixtures
- Solute
- Solution Properties Concentration
- Relation Between Molarity And Molality
- Osmotic Pressure Equation
Homogeneous Mixture Examples:
- The reaction between monoxide and O within the air
- The reaction between HCl and NaOH in water
- Oxy-acetylene torch burning
Heterogeneous reactions:
Heterogeneous reactions are chemical reactions within which the reactants and product are in two or a lot of phases. Therefore, any of the reactants and product is in one among the three sections: solid phase, liquid section, or gas section. Heterogeneous reactions lack uniformity. Moreover, the reactions that surface on the surface of a catalyst of a special section also are heterogeneous. These reactions are a lot more advanced because they contemplate the section of matter in conjunction with the character of interactions between the reactants.
Examples of heterogeneous reaction:
- Coal burning in air
- The reaction between salt and water
- Iron rusting underwater
- The reaction between sodium metal and water.
A homogenized mixture has a similar uniform look and composition throughout. several homogenized mixtures area unit normally remarked as solutions. Particle size distinguishes homogenized solutions from different homogeneous mixtures and heterogeneous mixtures. Solutions have particles that area unit the scale of atoms or molecules - too little to be seen. A mixture may be a homogenized answer with intermediate particle size between an answer and a suspension. A sugar answer is homogenized since solely a colorless liquid is discovered.
Air with no clouds is homogenized. A pure compound incorporates a constant composition with mounted ratios of parts. Though it's virtually physically not possible to isolate completely pure substances, a substance is alleged to be pure if no impurities may be detected by mistreatment, using the simplest accessible analytical techniques. Physical properties like boiling purpose or temperature of pure substances are unit invariant. As an example, pure water boils at a hundred degrees Anders Celsius.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
