Hybridisation: Definition, Formula, Examples, Questions, C2, BF3, Water
Have you ever thought about how carbon manages to form such a vast variety of compounds, from the soft graphite in pencils to the hardest diamond? How do atoms arrange their orbitals so perfectly to form stable bonds in different shapes? The answer is by hybridisation. Hybridisation is a process in which atomic orbitals mix to create new, equivalent orbitals that determine the shape and stability of molecules.
This Story also Contains
- Hybridization
- Types Of Hybridisation
- How To Find Hybridisation
- Some Solved Examples
- Practice More Question With The Link Given Below
- Summary

In this article, we will cover the concept of Hybridisation. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Hybridization
Hybridization means mixing atomic orbitals to recombine into a new set of hybrid orbitals. Types of hybridization include

The Salient Features And Conditions For Hybridization
-
Hybrid orbitals do not exist in isolated atoms. They are formed only in covalently bonded atoms.
-
Hybrid orbitals have shapes and orientations that are very different from those of the atomic orbitals in isolated atoms.
-
A set of hybrid orbitals is generated by combining atomic orbitals. The number of hybrid orbitals in a set is equal to the number of atomic orbitals that were combined to produce the set.
-
All orbitals in a set of hybrid orbitals are equivalent in shape and energy.
-
The type of hybrid orbitals formed in a bonded atom depends on its electron-pair geometry as predicted by the VSEPR theory.
-
Hybrid orbitals overlap to form σ bonds. Unhybridized orbitals overlap to form π bonds.
Also Read-
JEE Main- Top 30 Most Repeated Questions & Topics |
Most Scoring Concepts For JEE Mains 2025 April Session |
Most Scoring Concepts For JEE Mains 2025 January Session |
Types Of Hybridisation
The hybridization can be of several types depending on the number of hybrid orbitals involved in the formation of molecules. The table given below describes all types of hybridization and their geometries.

How To Find Hybridisation
The hybridization depends upon sigma bonds and a lone pair of electrons.
Thus,
Hybridization = Number of sigma bonds + Number of lone pairs present on the central atom
For example, hybridization for NH3 is sp3 and its molecular geometry is tetrahedral.
NH3 has 3 sigma bonds and 1 lone pair, thus hybridization for NH3:
3 sigma bonds + 1 lone pair = 4
Thus hybridization for NH3 is sp3 and its geometry is tetrahedral.
sp Hybridization
This hybridization process involves mixing of the valence s orbital with one of the valence p orbitals to yield two equivalent sp hybrid orbitals that are oriented in a linear geometry as shown in the figure. The number of atomic orbitals combined always equals the number of hybrid orbitals formed. The p orbital is one orbital that can hold up to two electrons. The sp set is two equivalent orbitals that point 180oC from each other. The two electrons that were originally in the s orbital are now distributed to the two sp orbitals, which are half filled.

When 1 s-orbital and 2 p-orbitals are involved in the molecule formation then the equivalent set of orbitals are known as sp2 hybrid orbitals. These hybrid orbitals arrange themselves at an angle of 120oC as shown in the figure.

When 1 s-orbital and 3 p-orbitals are involved in the molecule formation then the equivalent set of orbitals are known as sp3 hybrid orbitals. The bond angle between these hybrid orbitals is 109oC as shown in the figure.

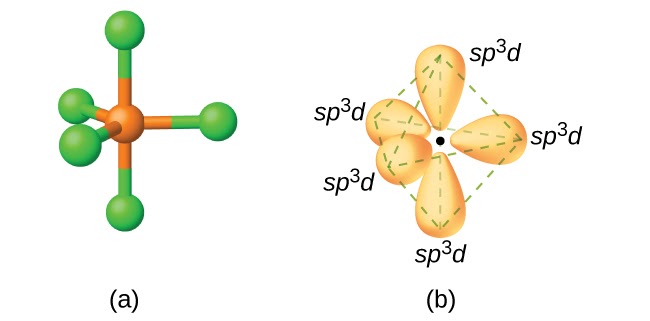
When 1 s-orbital, 3 p-orbitals, and 1 d-orbital are involved in the molecule formation then the equivalent set of orbitals are known as sp3d hybrid orbitals. There are two kinds of bonds formed for sp3d hybridization, i.e., 2 axial bonds and 3 equatorial bonds. The angle between the axial bond and the equatorial plane is 90oC while the bond angle between the equatorial bonds is 120oC as shown in the figure given below:
When 1 s-orbital, 3 p-orbitals and 2 d-orbitals are involved in the molecule formation then the equivalent set of orbitals are known as sp3d2 hybrid orbitals. There are two kinds of bonds formed for sp3d2 hybridisation, i.e., 2 axial bonds and 4 equatorial bonds. The angle between the axial bond and the equatorial plane is 90oC while the bond angle between the equatorial bonds is 90oC as shown in the figure given below:

When 2 d-orbital, 1 s-orbital and 3 p-orbitals are involved in the molecule formation then the equivalent set of orbitals are known as d2sp3 hybrid orbitals. There are two kinds of bonds formed for sp3d2 hybridisation, i.e, 2 axial bonds and 4 equatorial bonds. The angle between the axial bond and the equatorial plane is 90oC while the bond angle between the equatorial bonds is 90oC as shown in the figure given below:

When 1 s-orbital, 3 p-orbitals and 3 d-orbitals are involved in molecule formation then the equivalent set of orbitals are known as sp3d3 hybrid orbitals. The sp3d3 hybridization has a pentagonal bipyramidal geometry i.e., five bonds in a plane, one bond above the plane and one below it.

Related Topics:
Some Solved Examples
Example 1:
The type of hybridization and number of lone pair (s) of electrons of Xe in XeOF4, respectively, are :
1) sp3d2 and 1
2) sp3d and 2
3) sp3d2and 2
4) sp3d and 1
Solution
As we have learned in Hybridisation:

Hybridisation is sp3d2sp3 d2
It contains
It has 1 lone pair of electrons.
Hence, the correct answer is Option (1)
Example 2:
The correct statement about ICl5 and ICl4-is:
1)Both are isostructural
2)ICl5 is trigonal bipyramidal and ICl4-is tetrahedral
3)ICl5 is square bipyramidal and ICl4- is tetrahedral
4)ICl5 is square pyramidal and ICl4-is square planar.
Solution
ICl5 is square bipyramidal and Cl4−is square planar.
ICl5:−

Square Pyramidal
Lone Pair =1
Bond Pair=5
hybridisation= sp3d2
ICl4- :-

Square planar
Lone Pairs = 2
Bond Pairs = 4
hybridisation = sp3d2
Hence, the correct answer is Option (4)
Example 3:
The orbitals undergoing Hybridization involve
1) Orbitals of the same atom with almost similar energies
2)Orbitals of different atoms but with equal energies
3)Orbitals of different atoms with different energies
4)Orbitals of the same atoms with exactly equal energies
Solution
The orbitals of the same atom having similar energies undergo hybridization to form hybrid orbitals which have the same energy.
Hence, the answer is option (1).
Example 4:
In which pair of species, both species have a similar geometry?
1)CO2, SO2
2)CO23- and SO32−
3) SO42- and ClO4-
4)PH3 and BH3
Solution:
The geometry of CO2 is linear.
(O=C=O)
The geometry of SO2 is V-shape
The geometry of CO32- is trigonal planar.
The geometry of SO32− is a pyramidal shape
The geometry of SO42− is tetrahedral
The geometry of ClO4− is tetrahedral
The geometry of NH3 is a pyramidal shape
The geometry of BH3 is trigonal planar
Hence, the answer is option (3).
Example 5:
In
1)
2)
3)
4)
Solution:


 Hence, the correct answer is option (1).
Hence, the correct answer is option (1).
Practice More Question With The Link Given Below
Summary
Hybridization describes mixing atomic orbitals to form hybrid orbitals, which allows for the determination of the shape and bonding properties of molecules. It was introduced by Linus Pauling, and it includes types such as sp, sp2, sp3, sp3d, and sp3d2—each being associated with specific molecular geometries. For example, sp3 hybridization would give a tetrahedral shape and sp2 results in a trigonal planar structure. In forming hybrid orbitals, the 's' and 'p' orbitals of an atom combine, and sometimes the 'd' orbitals, thereby forming orbitals that become equal in energy and shape.
Also Check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Hybridisation is the process in which atomic orbitals of an atom mix (usually orbitals of similar energy) to form a set of new equivalent orbitals (hybrid orbitals).
It helps explain the geometry of molecules, bond angles, and the nature of bonds (sigma, pi) formed in many covalent compounds.
Typically orbitals of the same atom that have comparable energy levels (for example one s‐ and one or more p‐ orbitals) can undergo hybridisation. Fully filled or half‐filled orbitals may participate.
The greater the s‐character in the hybrid orbital, the shorter and stronger the bond formed, and the more electronegative the atom tends to be. For example, C–H bond in sp‐hybridised carbon is shorter & stronger than in sp³ carbon.
Count the number of sigma bonds + lone pairs around that atom → that gives approximate number of hybrid orbitals → match with type (2 → sp, 3 → sp², 4 → sp³, 5 → sp³d, 6 → sp³d² etc). Also consider the atom’s valence shell and presence of d‐orbitals if any.
No. It is not necessary for only half‐filled orbitals to participate. Even completely filled orbitals of slightly different energies can participate in hybridisation.
Yes. For example: In ethene (C=C) each C is sp² hybridised with one unhybridised p‐orbital forming the π bond. In ethyne (C≡C) carbon atoms are sp hybridised with two unhybridised p‐orbitals forming two π bonds.
Oxygen in H₂O is sp³ hybridised (one s + three p) giving four hybrid orbitals; two are used for bonding and two for lone pairs. The lone pair–lone pair and lone pair–bond pair repulsions reduce the bond angle from ideal 109.5° to ~104.5°.
Because questions often ask: hybridisation of a given atom in a molecule, geometry/bond angle prediction from hybridisation, effect of hybridisation on bond properties, and tricky cases (expanded valence, lone pairs, resonance). It appears frequently in past papers.
Hybridisation isn’t always simply the count of bonds: lone pairs matter.
For atoms in the third period or beyond, d‐orbitals may participate (expanded octet) → hybridisations like sp³d, sp³d².
Hybridisation is a model (from Valence Bond Theory) — some molecules may have resonance, delocalisation, or other bonding descriptions (e.g., MO theory) that complicate simple hybridisation assignment.
Correctly identifying the parent atom and counting all surrounding electron domains (bonds + lone pairs) is essential for correct hybridisation.