Isotonic, Hypertonic, Hypotonic Solution
The theory of solutions like isotonic, hypotonic, and hypertonic is very important in understanding the concept of cells. How cells react with their environment in terms of the balance of liquids and the osmotic pressure. The isotonic solution was discovered at the end of the 19th century as scientists began to understand the principles of osmosis and the scientist namely Jean-Baptiste Lamarck and William Cruickshank are the scientists who made significant contributions or made the framework for solution concentration and osmosis.
This Story also Contains
- Types of solution
- Some Solved Examples
- Summary
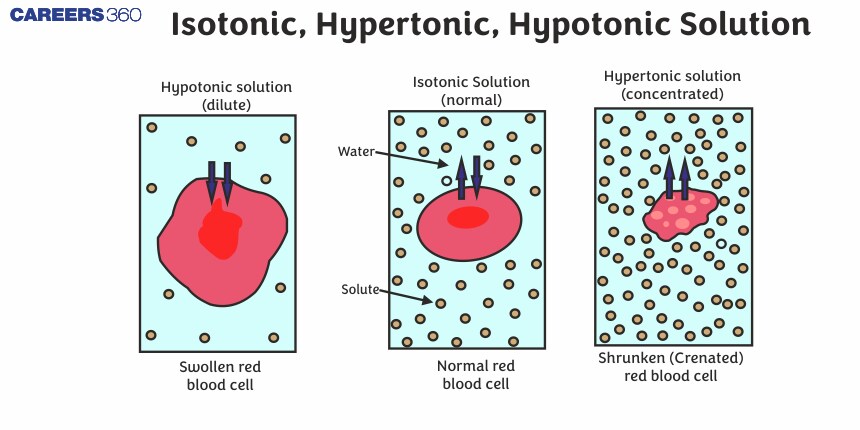
Hypertonic solutions are that solution which has a higher concentration of solute as compared to the inside of the cell. Which results in the water moving out of the cell and leads to the shrinkage of the cell. This was discovered by the scientists Johann Karl Wilhelm von Pfeffer and Wilhelm Röntgen in conjunction with the study of osmosis. Similar to isotonic and hyper tonic solutions the understanding of hypotonic solutions evolved as hypotonic solutions are that solution in which the solution has a lower solute concentration compared to the inside of the cell.
Types of solution
The solution is the homogenous mixture formed of two or more two substances. Basically it is composed of two substances the substances that are present in large quantities are called solvents and the substances that are present in smaller quantities are called solutes. The solution can have various states such as liquid solution, gaseous solution, and solid solution. All these are the states of solution but the types of solution are different these are as follows
- Isotonic solution
- Hypotonic solution
- Hypertonic solution
Isotonic Solution
Two solutions having the same osmotic pressure at a given temperature are called isotonic solutions. When such solutions are separated by semipermeable membrane no osmosis occurs between them. For example, the osmotic pressure associated with the fluid inside the blood cell is equivalent to that of 0.9% (mass/volume) sodium chloride solution, called normal saline solution and it is safe to inject intravenously.
Hypertonic Solution
The solution which has higher osmotic pressure is called a hypertonic solution. For example, if we place the cells in a solution containing more than 0.9% (mass/volume) sodium chloride, water will flow out of the cells and they will shrink. Such a solution is called hypertonic. It is a concentrated solution.
Hypotonic Solution
The solution which has lower osmotic pressure is called a hypotonic solution. For example, if the salt concentration is less than 0.9% (mass/volume), the solution is said to be hypotonic. In this case, water will flow into the cells if placed in this solution and they would swell. It is a diluted solution.

Recommended topic video on (Isotonic, Hypertonic and Hypotonic Solution)
Some Solved Examples
Example.1
1. Which of the following is more hypertonic than 1 M Glucose solution?
1)1 M urea
2)0.5 M NaCI
3) (correct)0.75 M KCl
4)None of these
Solution
Hypertonic Solutions -
A solution having osmotic pressure more than another
wherein
If $\pi_1>\pi_2$ Then solution 1 is hypertonic osmotic pressure $\pi$
$\pi$ of Glucose $=1 \times R T$
$\pi$ of urea $=1 \times R T$
$\pi$ of NaCl $=2 \times 0.5 R T=1 \times R T$
$\pi$ of KCl $=2 \times 0.75 R T=1.5 \times R T$
The osmotic pressure of the KCl solution is higher than the glucose solution, So it is more hypertonic than the glucose solution.
Hence, the answer is the option (3).
Example.2
2. Which of the following is hypertonic than 1m NaCl solution?
1)1m $\mathrm{CaCl}_2$ Solution
2)1m AgCl Solution
3)1m $A l_2\left(\mathrm{SO}_4\right)_3$ Solution
4) (correct)(a) & (c) both
Solution
Hypertonic Solutions -
The solution has osmotic pressure more than the other
If $\pi_1>\pi_2$
Then solution 1 is hypertonic
If molarity and temperature are constant then the osmotic pressure depends on Vant Hoff's factor since Vant Hoff factor of CaCl2 and $\mathrm{Al}_2\left(\mathrm{SO}_4\right)_3$ is higher than NaCl both can form a Hypertonic solution than NaCl.
Hence, the answer is the option (4).
Example.3
3. Which of the following is hypertonic with 1 M CaCl2 solution?
1)1 M Urea Solution
2)1 M Glucose Solution
3)1 M NaCl Solution
4) (correct)None of the above
Solution
Vant Hoff factor for compounds are:
Compound NaCl CaCl2 Glucose Urea | Vant Hoff factor 2 3 1 1 |
So, CaCl2 has 3, and others have different.
Hence, the answer is the option (4).
Example.4
4. A 0.6% solution of urea (molecular weight = 60) would be isotonic with:
1) (correct)0.1 M glucose
2)0.1 M KCl
3)0.6% glucose solution
4)0.6% KCl solution
Solution
Isotonic solutions have the same osmotic pressure
$\begin{aligned} & \pi_1=\pi_2 \\ & C_1 T_1=C_2 T_2\end{aligned}$
At constant Temperature
$C_1=C_2$
Isotonic solutions are those which have the same concentration.
0.6% urea solution has 0.6 g of urea dissolved in 100 ml of solution.
6 g of urea will be present in 1000ml of solution
Thus, C = 0.1M.
Now, 0.1 M solution of glucose will be isotonic with the given urea solution
Hence, the answer is the option (1).
Example.5
5. Which of the following solutions is hypotonic to 0.1 M solution of Urea?
1)0.1 M Glucose solution
2)1.8 w/v % solution of Glucose
3) (correct)0.9 w/v% solution of Glucose
4)0.6 w/v% solution of Urea
Solution
The given solution of Urea is 0.1 M. Hence, a solution that is hypotonic to this solution of urea must have a concentration less than 0.1 M.
Now,
0.9% w/v solution of glucose contains 0.9 g in 100ml solution
$\therefore$ 1000ml solution contains 9 g of Glucose
$\therefore$ Molarity $=\frac{9}{180 \times 1}=0.05 \mathrm{M}$
Hence, the answer is the option (3).
Example.6
6. A 5.25% solution of a substance is isotonic with a 1.5% solution of urea (molar mass = 60 g mol-1) in the same solvent. If the densities of both the solutions are assumed to be equal to 1.0 g cm-3, the molar mass (in g/mol) of the substance will be
1) (correct)210
2)90
3)115
4)105
Solution
Isotonic solutions have the same osmotic pressures.
$\begin{aligned} & \pi_1=\pi_2 \\ & C_1 T_1=C_2 T_2\end{aligned}$
at constant Temp.
$\begin{aligned} & C_1=C_2 \\ & \frac{1.5 / 60}{V}=\frac{5.25 / \text { Molecular Mass }}{V} \\ & M=\frac{5.25 \times 60}{1.5}=210\end{aligned}$
Hence, the answer is the option (1).
Summary
Isotonic, hypotonic, and hypertonic solutions are very important each plays a very important role in balancing the osmosis of biological and chemical systems or the system of medical treatments. The isotonic solution has various applications like in the medical setting for intravenous fluids in which this solution has to maintain the fluid shift and maintain the integrity of cells. The example of the isotonic solution includes 0.9% saline solution and The hypertonic solution contains a higher concentration of solute than the solution. the hypertonic solution is used to treat the edema. Edema is the swelling of the tissues. And hypertonic solution treat it by drawing excessive fluid out of the tissue. For example, 3% of the saline solution is used to treat severe hyponatremia.