States of Matter (Solid, Liquid, Gas) Definition, Classification FAQs
Introduction to Matter
What is matter in science?
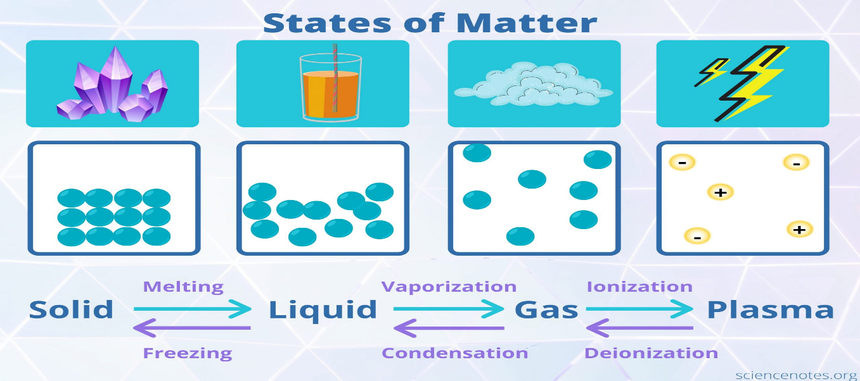
The matter is explained as anything that has mass and volume (occupies space)". Matter can be defined as the substance which constitutes the observable universe. Matter, along with energy, is known to form the basis of every objective phenomenon. In classical physics and general chemistry, the matter is applied to determine any material that contains mass and occupies volume. The matter is composed of several atoms or molecules. The different arrangements of these atoms or molecules give matter various states and properties. The force of interaction between these particles provides matter its physical states, based on which matter can be classified into solid, liquid, and gas mains. The force of interaction between atoms/molecules is highest in solids, least in gas, and intermediate in gas.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Introduction to Matter
- Types of matter: (What are the states of matter?)
- Solid matter
- Properties of solid, liquid, and gas with examples
- Liquid state of matter
- Gas state of matter
- plasma state of matter
- Bose-Einstein Condensates
- What are solid liquid and gas?
In this unit, we will learn more about these three physical states of matter particularly – solid, liquid, gas.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Types of matter: (What are the states of matter?)
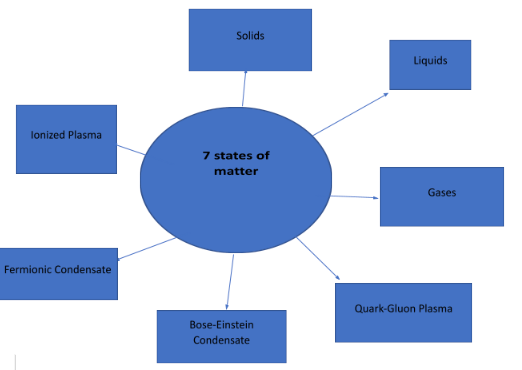
7 states of matter
Solid matter
(Solid state of matter)
Solid Definition (solid definition chemistry)
A solid can be defined as a substance that occupies a specific volume in a very rigid form hence, does not change its shape. This rigidity achieves due to the closely packed atoms whose kinetic energies are much lower than those of liquids and gases.
Properties of solid, liquid, and gas with examples
In solids, particles are tightly or closely packed due to the strongest intermolecular force of interaction.
The gaps between the particles are very small and therefore, tough to compress. As a result, Solid has a fixed shape and volume.
Because of the rigid nature of the solid, particles in the solid can only vibrational motion along with their mean position and cannot move.
The force of attraction between particles is obstinate.
In solids, the diffusion rate is very small.
Example of Solid: solid ice, sugar, rock salt, vapour, etc.
These are the properties of solid liquid and gas.
Three-Dimensional Lattice
The unique and special arrangement of atoms or molecules within a crystal is referred to as the lattice structure of that material. The 3-D lattice structure shows the periodic arrangement of the atoms which forms a crystalline substance. Crystalline materials are so highly systematic that a crystal lattice is formed from repetitions along all three spatial dimensions of the same pattern. The crystal lattice represents the three-dimensional structure of the atomic/molecular components of the crystal.
The Unit Cell
The structure seen within the crystalline lattice of a material can be described in a different way. The most common way to describe a crystal structure is to refer to the size and shape of a characteristic unit cell of matters, i.e., the simplest repeating unit within the crystal. These unit cells repeat to create a three-dimensional lattice.
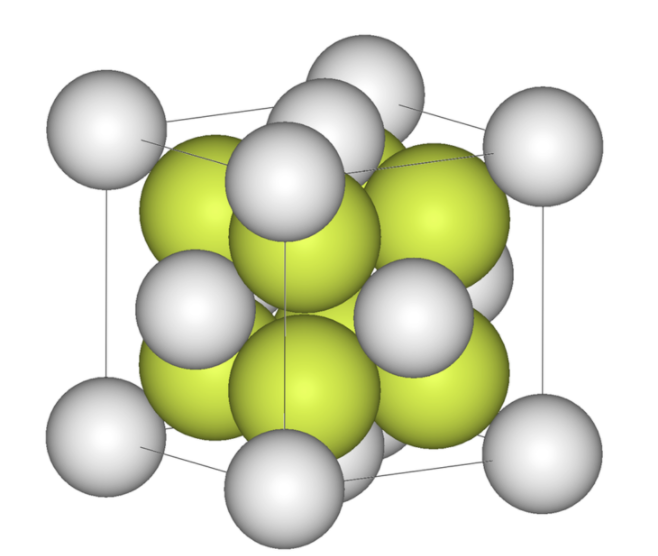
Unit Cell of Fluorite: the above diagram shows the simplest repeating unit within a crystal of the molecule calcium fluoride (CaF2). Calcium ions are indicated as grey spheres, and fluorine ions are indicated as yellow.
Packing of Atoms Within a Unit Cell
Within a crystalline material, each atom can be considered as a sphere that is packed into unit cells. Each and every sphere that is present in a crystal structure consists of a coordination number, which corresponds to the number of spheres within the crystalline structure that touches the sphere that is being evaluated.
Liquid state of matter
liquid definition.
It is composed of molecules or atoms in such a way so that they can move freely among themselves but do not tend to separate like gases and it occupies the shape of the container in which it is kept,
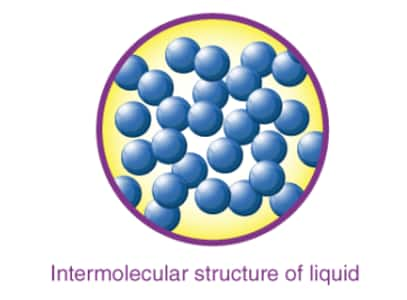
Characteristics of liquid state.
In a liquid matter, particles are less tightly packed than solid.
Liquids occupy the shape of the container in which they are kept.
Liquids are difficult almost incompressible as particles have less intermolecular space to move.
Liquids have fixed volume but do not possess any particular shape.
The rate of diffusion in liquids is higher than that of solids but lesser than that of gas.
The Force of attraction between the particles is weaker than solids but higher than that of gas.
Liquid exhibits some properties like viscosity and surface tension.
Example of a liquid state of matter/ Examples of liquid/ examples of liquids: water, milk, blood, coffee, etc.
Related Topics, |
Types of liquids
Liquids may be divided into two general types:
pure liquids and liquid mixtures.
Diffusion of solids in liquids
Diffusion is a phenomenon in which substances move from an area of high concentration to an area of low concentration.
The movement of the molecule takes place from the area of higher concentration to the area of lower concentration until it becomes the same. Liquid molecules and gases molecule undergo diffusion as they move freely. The process diffusion is based on the concentration gradient.
Solid can diffuse in liquid. When sugar is added to water, whole water becomes sweet without stirring it because of the diffusion of sugar into the water.
Gas state of matter
Definition of gas state.
A state of matter in which it will expand freely to fill the whole of a container, having no fixed shape (unlike a solid) and no fixed volume (unlike a liquid).
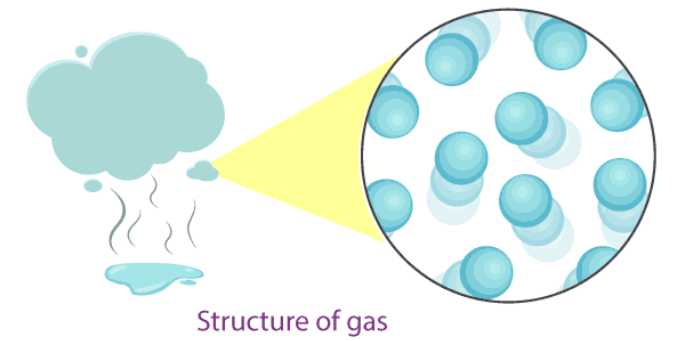
Characteristic of gas
In this state of matter, the intermolecular distances are large (in the range of 10−7–10−5cm10−7–10−5cm).
The intermolecular Force of attraction between the particles is negligible, and they can move freely.
Gases have neither a fixed volume nor a fixed shape, hence, occupies the shape of the container.
The compressibility is higher in gases as compared to solids and liquids.
The diffusion rate is higher in gases than solids and liquids.
The kinetic energy of particles is higher than in solids and liquids.
Gases example include air, nitrogen, helium, oxygen, carbon dioxide, etc.
pictures of different gases:
plasma state of matter
What is plasma in chemistry?
Plasma is the fourth state of matter known as the superheated state of matter. The plasma includes 99% of the universe which is visible.
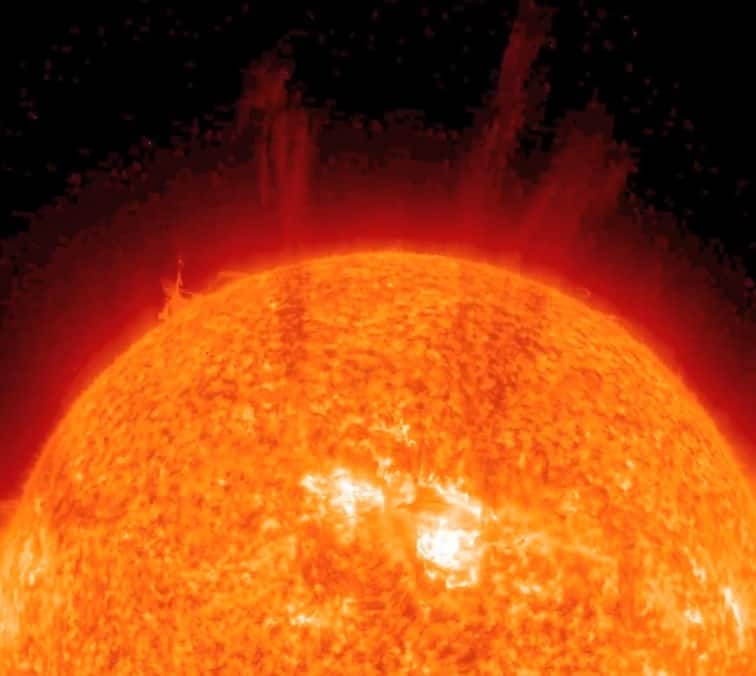
Properties of plasma state
Plasma behaves like an intermediate state of gas and liquid
plasmas show no fixed shape or volume. It is denser than solids or liquids.
"Plasma is a charged gas, with strong Coulomb [or electrostatic] interactions,"
Plasma consists of particles that possess extremely high kinetic energy.
Plasma is used in the formation of the sun and stars.
Examples of plasma: sun, stars, lightning, solar wind, fusion devices, welding arcs, neon signs, aurora, nebulae, galaxies, etc.
Also, students can refer,
- NCERT solutions for Class 11 Chemistry Chapter 5 States of Matter
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 5 States of Matter
- NCERT notes Class 11 Chemistry Chapter 5 States of Matter
Bose-Einstein Condensates
The 5th state of matter is Bose-Einstein Condensates.
It is defined as a state of matter in which separate atoms or subatomic particles, cooled to near absolute zero temperature (0 K or, − 273.15 °C).
Characteristics:
Bose-Einstein condensates of matter were made with the help of advanced technology.
Carl Weiman and Eric Cornell froze a sample of rubidium with the help of magnets and lasers to within a few degrees of absolute zero.
At that temperature, the motion of the molecules becomes negligible or almost tends to zero. As this lowers the kinetic energy, the atoms no longer stay separated and begin to clump together. Since the atoms join together, they form a super-atom.
Light slows down as it passes through a Bose-Einstein condensate of matter, helping scientists to study more about the nature of light as a wave and particle.
Bose-Einstein condensates also show properties of a superfluid which implies, it flows without friction.
What are solid liquid and gas?
The principal physical states of matter are solid liquid and gas.
Solid: A solid can be defined as a substance that occupies a specific volume in a very rigid form hence, does not change its shape. This rigidity achieves due to the closely packed atoms whose kinetic energies are much lower than those of liquids and gases. Liquid: composed of molecules or atoms in such a way so that they can move freely among themselves but do not tend to separate like gases and it occupies the shape of the container.
Gas: a state of matter in which it will expand freely to fill the whole of a container, having no fixed shape (unlike a solid) and no fixed volume (unlike a liquid).
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Solid liquid gas
Bose-Einstein Condensates.
There are 7 states of matter;
Solids, Gases, Liquids, Ionized Plasma, Bose-Einstein Condensate, and Fermionic Condensate, Quark-Gluon Plasma.
Plasma is an ionized gas and the fourth state of matter.
1] Both liquids and solids have definite shapes.
2] Both liquids and solids are formed of molecules.
In physics, matter can be defined as the quantity having mass and occupying space and it makes the whole universe.
Plasma
Also Read
11 Mar'25 12:08 PM
19 Feb'25 11:49 AM
19 Feb'25 11:23 AM
19 Feb'25 11:14 AM
19 Feb'25 10:31 AM
18 Feb'25 12:15 PM
18 Feb'25 11:59 AM
09 Feb'25 10:16 PM
08 Feb'25 05:31 PM
08 Feb'25 05:28 PM