Mendeleev used Sanskrit prefixes eka‑ (one), dvi‑ (two), and tri‑ (three) to temporarily name predicted elements based on their positions relative to known ones—for instance, eka‑silicon was the element directly below silicon. He also admired the two-dimensional structure of the Sanskrit alphabet (varṇamālā)—which grouped sounds by pronunciation features—and saw a parallel in his periodic arrangement of elements, honoring ancient grammarian Pāṇini with this naming approach.
Mendeleev’s Periodic table
Dmitri Mendeleev's formulation of the Periodic Table in 1869 stands as a pivotal advancement in the field of chemistry, profoundly altering our comprehension of elemental relationships. By systematically arranging elements in order of increasing atomic mass, Mendeleev discerned recurring patterns in their chemical properties, leading to the establishment of the periodic law. This innovative approach not only facilitated the classification of known elements but also enabled the prediction of properties for undiscovered ones. His work continues to be a cornerstone in the study of chemistry, underscoring the significance of periodicity in elemental properties.
This Story also Contains
- Mendeleev's Periodic Table: Pioneer to the arrangement of elements.
- Correction of doubtful atomic weights:
- Defects of Mendeleev's Periodic Table:
- Lother Meyer:
- Solved Examples Based On Mendeleev's Periodic Table
- Conclusion
This topic is integral to the Class 11 Chemistry curriculum under the Classification of Elements and Periodicity in propeties chapter. Understanding Mendeleev's contributions is crucial not only for academic examinations but also for competitive assessments such as JEE Main, NEET, and other entrance tests. Notably, the concept was featured in the JEE exam in 2021, highlighting its enduring relevance in the field of chemistry.
Mendeleev's Periodic Table: Pioneer to the arrangement of elements.
Dmitri Mendeleev:
Dmitri Mendeleev's formulation of the Periodic Law and his development of the Periodic Table marked a transformative moment in chemistry. At a time when the structure of atoms was not yet understood, Mendeleev's insight that the properties of elements are related to their atomic masses was groundbreaking. While constructing the table, he arranged elements by increasing atomic mass and grouped them based on similar chemical properties. Recognizing that some elements did not fit this arrangement, Mendeleev took the bold step of reversing the order of certain elements to maintain consistency in the table's structure.
Moreover, Mendeleev demonstrated remarkable foresight by leaving gaps in his table for elements that were not yet discovered. He predicted the properties of these missing elements based on the trends he observed among known elements. His predictions proved astonishingly accurate upon the discovery of these elements. For instance, he predicted the existence of gallium, which was discovered in 1875, and germanium, discovered in 1886, both of which exhibited properties closely matching Mendeleev's forecasts .
Mendeleev's Periodic Law not only organized the known elements but also provided a predictive framework that spurred further research and discovery in the field of chemistry. His work laid the foundation for the modern periodic table and continues to influence scientific thought and education today.
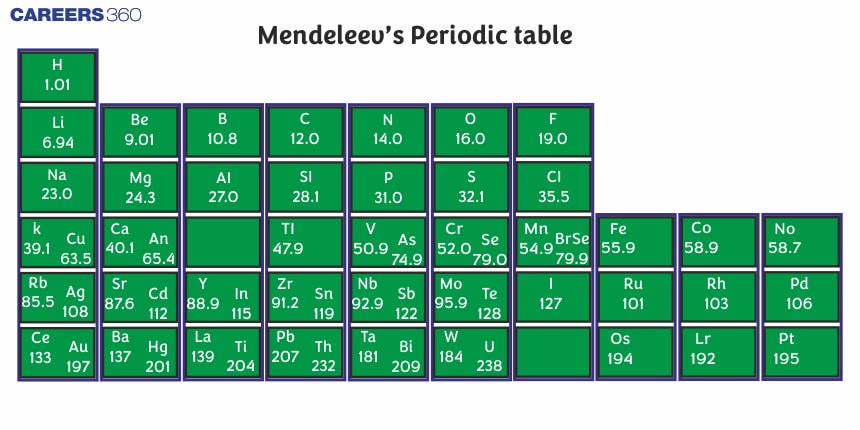
Mendeleev's Periodic Table:
Mendeleev's periodic law: The physical and chemical properties of elements are the periodic function of their atomic weight
Characteristic of Mendeleev's periodic table :
It is based on atomic weight
63 elements were known, but noble gases were not discovered.
He was the first scientist to classify the elements in a systematic manner i.e. in horizontal rows and vertical columns.
Horizontal rows are called periods and there were 7 periods in Mendeleev's Periodic table.
Vertical columns are called groups and there were 8 groups in Mendeleev's Periodic table.
Each group up to VIIth is divided into A & B subgroups.'A' sub-group elements are called normal elements and 'B' sub-group elements are called transition elements.
The VIIIth group consisted of 9 elements in three rows (Transitional metals group).
The elements belonging to the same group exhibit similar properties.
- Merits or Advantages of Mendeleev's periodic table :
- Study of elements: First time all known elements were classified according to their similar properties. So study of the properties of elements becomes easier.
Prediction of new elements: It encouraged the discovery of new elements as some gaps were left in it.
Sc (Scandium) Ga (Gallium) Ge (Germanium) Tc (Technetium)
These were the elements for whom position and properties were well defined by Mendeleev even before their discoveries and he left the blank spaces for them in his table.
Ex. Blank space at atomic weight 72 in the silicon group was called Eka silicon (which means properties like silicon) and the element discovered later was named Germanium.
Similarly, other elements discovered after Mendeleev's periodic table were.
Eka aluminium – Gallium(Ga)
Eka Boron – Scandium (Sc)
Eka Silicon – Germanium (Ge)
Eka Manganese – Technetium (Tc)
Correction of doubtful atomic weights:
Correction was done in the atomic weight of some elements.
Atomic weight = Valency × Equivalent weight.
Initially, it was found that the equivalent weight of Be is 4.5 and it is trivalent (V = 3), so the weight of Be was 13.5 and there is no space in Mendeleev's table for this element. So, after correction, it was found that Be is divalent (V = 2). So, the weight of Be became 2 × 4.5 = 9 and there was a space between Li and B for this element in Mendeleev's table.
– Corrections were done in the atomic weight of elements are – U, Be, In, Au,
Defects of Mendeleev's Periodic Table:
The position of hydrogen is uncertain. It has been placed in the lA and VII-A groups because of its resemblance with both groups.
No separate positions were given to isotopes.
It is not clear whether the lanthanides and actinides are related to IIA or IIB group.
Although there is no resemblance except the valency of subgroups A and B, they have been put in the same group.
The order of increasing atomic weights is not strictly followed in the arrangement of elements in the periodic table. E.g. – Co (At. wt. 58.9) is placed before I (127) and Ar (39.9) before K (39).
Lother Meyer:
He plotted a curve between the atomic weight and the atomic volume of different elements.
The following observations can be made from the curve –
Most electropositive elements i.e. alkali metals (Li, Na, K, Rb, Cs, etc.) occupy the peak positions on the curve.
Less electropositive i.e. alkali earth metals (Be, Mg, Ca, Sr, Ba) occupy the descending position on the curve.
Metalloids (B, Si, As, Te, At, etc.) and transition metals occupy the bottom part of the curve.
Most electronegative i.e. halogens (F, Cl, Br, I) occupy the ascending position on the curve.
Note: Elements having similar properties occupy similar positions on the curve.
Based on this curve Lother Meyer proposed that the physical properties of the elements are periodic functions of their atomic wt. and this becomes the base of Mendeleev's periodic table.

Also Read:
- Development Of Modern Periodic Table
- Modern Periodic Table
- Electronic Configuration In Periods And Groups
Recommended topic video on ( Mendeleevs Periodic table)
Solved Examples Based On Mendeleev's Periodic Table
Example 1: Eka-aluminium and Eka-silicon are respectively known as:
1) Aluminium and silicon
2) Silicon and aluminium
3) Gallium and germanium
4) Gallium and Tin
Solution:
According to Mendeleev’s periodic table, Eka-aluminium is known as gallium and Eka-silicon is known as germanium.
Hence, the answer is the option (3).
Example 2: Mendeleev’s periodic table is based on:
1) Atomic number
2) Atomic weight
3) Ionization enthalpy
4) None of the above
Solution
Mendeleev arranged the elements in horizontal rows and vertical columns in his table in order of their increasing atomic weights. In this way, elements with similar properties occupied the same vertical column.
Hence, the answer is the option (2).
Example 3: Which of the following statements is not correct about Lother Meyer’s curve?
1) The alkali metals occupy the maxima of the curve
2) The transition metals occupy the minimum of the curve.
3) The halogens occupy positions on the descending portions of the curve.
4) The alkali earth metals occupy mid positions on the descending portions of the curve.
Solution: The Lother Meyer curve. Thus, halogens occupy positions on the ascending portions of the curve.
Therefore, Option(3) is correct.
Practice more Questions from the link given below:
Conclusion
Thanks to the great genius of one of the most outstanding chemists in history, mankind has acquired a unique publication, the Periodic Table of Mendeleev. His way of organizing the elements might be great because not only offered a system of knowing the properties of the elements but also opened doors for further interpretations and breakthroughs in chemistry. In the way that he guessed at and calculated the probable location and characteristics of still undiscovered elements, Mendeleev proved anew the fruitfulness of the mental exercise of rational processes complemented by the observation of the facts. Nevertheless, the further elaboration and modifications, which have been made to the Periodic Table by other chemists, people remember the genius of Mendeleev’s work, which played a crucial role in the formation of contemporary chemistry.
Frequently Asked Questions (FAQs)
Mendeleev’s periodic table struggled to place hydrogen logically, forced some heavier elements before lighter ones to maintain group traits (like Co/Ni or Te/I), and couldn’t address isotopes, noble gases, or f‑block elements due to its reliance on atomic mass.
Mendeleev’s table (1869) arranged 63 known elements by increasing atomic mass, grouped similar properties, and left gaps predicting elements like gallium and germanium .
Modern periodic table orders all 118 elements by atomic number, includes noble gases and transition blocks, and naturally resolves earlier anomalies without gaps .
Mendeleev's Periodic Table is an arrangement of chemical elements organized by increasing atomic mass. Proposed by Dmitri Mendeleev in 1869, it grouped elements with similar chemical properties into columns, revealing periodic trends in their behaviors.
Mendeleev arranged elements in rows (periods) and columns (groups) based on their atomic masses. He placed elements with similar properties in the same vertical columns, leaving gaps for elements that were yet to be discovered.
Mendeleev's Periodic Law states that "the physical and chemical properties of elements are periodic functions of their atomic masses." This means that elements show recurring trends in properties when arranged by increasing atomic mass.
Yes, Mendeleev left gaps in his periodic table for elements that had not yet been discovered. He predicted the properties of these missing elements, and many were later discovered, confirming his predictions.
In cases where elements with similar properties had different atomic masses, Mendeleev sometimes placed them out of order to maintain the consistency of chemical properties within groups. For example, he placed iodine before tellurium, despite iodine having a higher atomic mass, because iodine's properties were more similar to those of other halogens.
