Modern Periodic Table Modern Periodic Law - Definition, Examples, Properties, FAQs
Define The Modern Periodic Table:
The modern periodic table is given by properties of elements are determined by the periodic functions of their atomic numbers, according to the current periodic law. Across each row, elements were arranged from left to right in increasing order of their atomic numbers. There is a regular pattern in the elements with similar properties. the modern periodic table is based on atomic numbers.
Why Atomic Number Instead of Atomic Mass?
There are protons and neutrons in every nucleus of an atom, and their mass is referred to as atomic mass. A nuclear nucleus has a specific number of protons, whereas an atomic number indicates the number of those protons. In addition, there are exactly as many protons in the nucleus as electrons outside it.
The nucleus of an atom is embedded deep within the atom. There are some electrons outside of it, especially those in the outermost shell, which can move freely. Chemical reactions are therefore involved in their activity. As a result, the properties of an element depend on its atomic number rather than its atomic mass.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
The Long Form of the Periodic Table
According to the modern periodic law, the modern periodic table is based on the long-form. A periodic table is an arrangement of elements in increasing order of their atomic number.
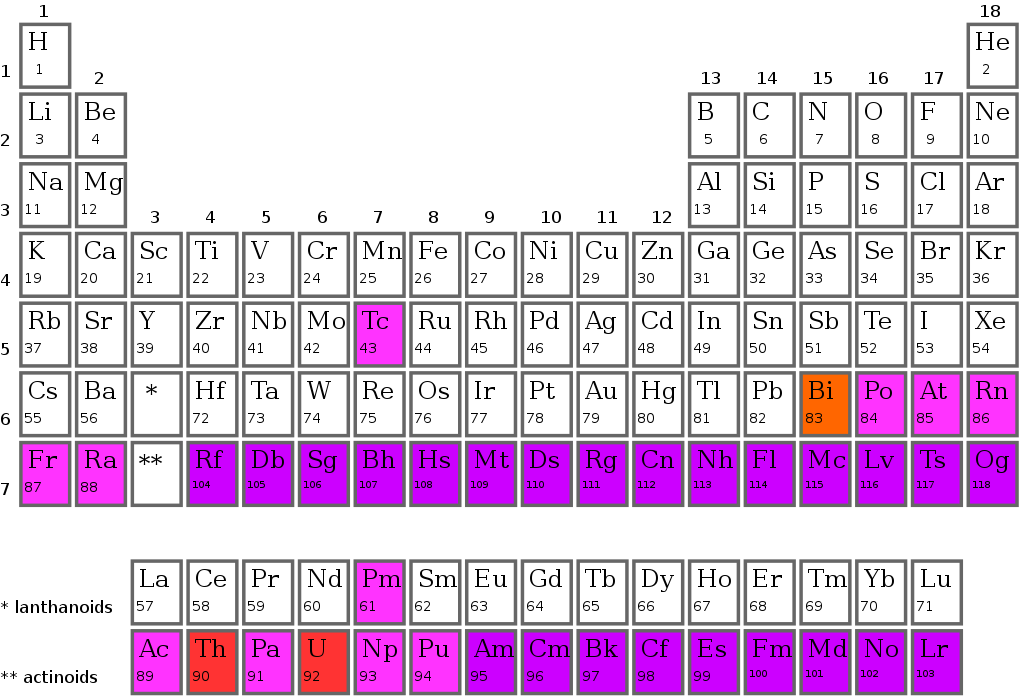 The Periodic Table
The Periodic Table
Also read :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Periodic groups in the the modern periodic table
The modern periodic tables have vertical columns called groups. In the periodic table, there are 18 groups. one through eighteen groups is numbered. The elements in each group have the same outer shell electronic configuration. The Periodic Table of the Modern Era Whenever you see a horizontal row in the modern or long periodic table, it is a period.
The periodic table has seven periods. There are 1, 2, 3, 4, 5, 6, and 7 on each side. One of the elements in the first period is hydrogen. The other element is helium. Two of the three periods consist of eight elements each. There are 18 elements in each of the 4th and 5th periods The 6th period, however, consists of 32 elements.
In the seventh period of the periodic table, four new elements have been discovered. Nihonium 113, Meconium 115, Tennessine 117, and Magnesium 118. There are 32 elements in this addition, completing the 7th period. There is an additional panel at the bottom of the long periodic table. Lanthanides are 14 elements of the 6th period. The 7th period of the periodic table has 14 elements called actinides. The number of shells or energy levels in the atom of an element corresponds to its period.
The modern periodic table: The Cause of Periodicity
Regular repetitions of similar outer electron configurations are thought to explain periodicity in properties. The outer electronic configuration of all group 1 elements, the alkali metals, is the same, ns1.
In this example, n refers to the Principal Quantum Number of the outermost shell. The outer electronic configuration of elements in group 17 is ns2 np5. As a result, they are similar in their properties. Halogens are these substances.
A similar outer electronic configuration is found for elements of group 18. The orbits are filled to the brim. Known as inert gases, they are nonreactive elements. Argon and Helium are also members of this group due to their electron configurations. Inert gases are the ones that are together and are known as such!
Element properties within a group are similar to those of all the other members of the same group. Their outer shell electron configurations are all similar.
Modern periodic law: What is it?
Mendeleev developed a periodic table based on the periodic law and the periodic law. Mendeleev developed the periodic table toward the end of the 18th century. In the old days, scientists weren't aware of the atom's internal structure. The atomic number is the most basic property of a chemical element, as revealed by the development of various atomic models and advancements in quantum theory. Mendeleev's periodic law was subsequently modified, resulting in what is now called a modern periodic theory.
NCERT Chemistry Notes:
Definition of the modern periodic law
Modern periodic law can be summarized as follows:
Periodic functions of the atomic numbers of the elements determine their physical and chemical properties."
Neutral atoms contain electrons or protons whose number depends on their atomic structure. The fundamental unit of element knowledge led scientists to understand quantum numbers and the electronic configuration of elements on the periodic table. Chemistry discovered that the 94 naturally occurring chemical elements have analogies based on the periodic law. A comparison between these elements caught people's attention. Many synthetic elements were created by scientists. Modern periodic law-based periodic tables were derived from Mendeleev's periodic table.
Associated Terms:
There is a total of protons in the nucleus of an atom of an element that is its atomic number.
An element's mass number is the number of protons and neutrons inside the nucleus of the atom of that element.
The atomic mass of an atom, subatomic particle, or molecule is called its atomic mass.
Atomic or molecular orbitals define an atom or molecule's electronic configuration.
This temperature is used as part of the melting point of an element to change it from solid to liquid.
In a liquid, the boiling point occurs when the vapor pressure equals the pressure around the liquid. The temperature at which liquid turns into vapor is called the vaporization point.
Also check-
