Modern Periodic Table - Introduction, Names, Trend, FAQs
Modern Periodic Table: Introduction, History.
The periodic table, often known as the periodic table of (the) elements, is a table that shows the chemical elements in a tabular format. It's commonly used in chemistry, physics, and other sciences, and it's considered a chemistry icon. It is a graphic representation of the periodic law, which claims that the properties of chemical elements are related to their atomic numbers in a predictable manner.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Modern Periodic Table: Introduction, History.
- What is the Modern Periodic Table?
- Periodic Trend
The table is separated by four blocks, which are roughly rectangular sections. The table's rows are known as periods, while the columns are known as groups. Chemical properties of elements belonging to the same periodic table column group are comparable. Non-metallic character (holding their own electrons) way across the periodic table. The underlying cause of these patterns is atom-electron configurations.
The first widely regarded periodic chart was created by Russian chemist Dmitri Mendeleev in 1869, who developed the periodic law as a relationship between chemical qualities and atomic mass. There were gaps in Mendeleev's periodic table since not all elements were known at the time, and he was able to anticipate attributes of some of the missing elements using the periodic law.
The periodic law was considered an important discovery in the late nineteenth century, and it was explained by the atomic number discovery and early twentieth-century quantum mechanics studies that exposed the atom's internal structure of the Modern Periodic Table.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
What is the Modern Periodic Table?
The Modern Periodic Table, often known as the Periodic Table, is a table that classifies chemical elements by their atomic numbers, electron configurations, and chemical characteristics of the Modern Periodic Table. The “S”, “P”, “D”, and “F” blocks are the four blocks that make up a Modern Periodic Table of elements. Non-metallic elements are found on the right side of the Modern Periodic Table of Elements, while metallic elements are found on the left.
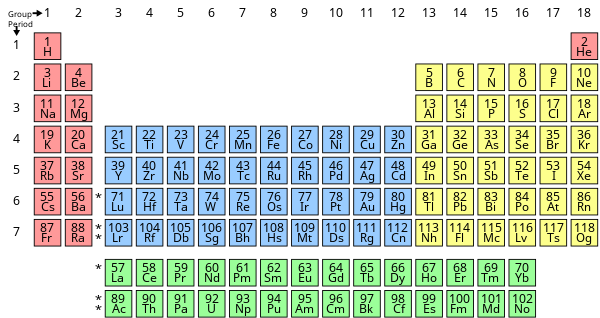
Periodic table groups:
In chemistry, a group is a column in the Modern Periodic Table of elements of chemical elements. Atoms in a group of chemical elements have the same number of valence electrons and valence vacancies. Because their atomic valence shells are similar in composition and structure to the Modern Periodic Table, they have chemical and physical properties that are comparable as well.
The groups are numbered one through eighteen. The s-block, or hydrogen block, of the periodic table contains two groups (1 and 2) of elements; the d-block, or transition block, contains ten groups (3 through 12); and the p-block, or main block, contains six groups (13 through 18). The lanthanoids and actinoids in the f-block, or inner-transition block, are not assigned group numbers.
Also read :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Periodic table periods:
All chemical elements discovered or created are listed in the periodic table of the elements, which is divided into seven horizontal periods in order of their atomic numbers, with the lanthanoids (lanthanum, 57, to lutetium, 71) and actinoids (actinium, 89, to lawrencium, 103) listed separately below. The lengths of the periods vary. The first is the hydrogen period, which is made up of the two elements hydrogen and helium. Then there are two eight-element periods, the first of which runs from lithium 3 to neon 10 and the second of which runs from sodium 11 to argon 18.
The first, from potassium 19 to krypton 36, and the second, from rubidium 37 to xenon 54, are both extended periods with 18 elements each. The omission of the lanthanoids (which are listed separately below) condenses the first very long period of 32 elements, from cesium, 55, to radon, 86, into 18 columns, allowing the remaining 18 elements, which have properties that are very similar to those of the first and second long periods, to be placed directly below them.
Related Topics Link |
Periodic Trend
Trends in Electronegativity
1. Electronegativity grows from left to right across a period of components. When an atom's valence shell is less than half full, losing an electron takes less energy than gaining one. It is easier to pull an electron into the valence shell than to contribute one if the valence shell is more than half full.
2. Electronegativity falls from the top to the bottom of a group. Because the distance between the valence electrons and the nucleus increases as the atomic number falls, the atomic radius increases.
3. The noble gases, lanthanides, and actinides are notable exceptions to the preceding criteria. Noble gases have a full valence shell and do not normally attract electrons. The chemistry of the lanthanides and actinides is more intricate and does not generally follow any patterns. As a result, electronegativity values do not exist for noble gases, lanthanides, and actinides.
4. Despite the fact that transition metals have electronegativity values, there is little variation between them over time and within groups. This is due to the fact that their metallic qualities interfere with their capacity to attract electrons as readily as other elements.
Ionization Energy Trends
1. The ionization energy of the elements increases from left to right within a period. This is owing to the stability of the valence shell.
2. From top to bottom, the ionization energy of the components within a group drops. Electron shielding is to blame for this.
3. Due to their complete valence shells, noble gases have extremely high ionization energies, as shown in the graph. The ionization energy of helium is the highest of all the elements.
NCERT Chemistry Notes:
Electron Affinity Trends
1. Electron affinity shifts from left to right over time. The decrease in the atomic radius is the reason for this.
2. Within a group, electron affinity diminishes from top to bottom. The rise in the atomic radius is to blame.
Atomic Radius Trends
1. Within a period, the atomic radius decreases from left to right. The rise in the number of protons and electrons over time causes this. Because one proton has a stronger influence than one electron, electrons are drawn towards the nucleus, causing the radius to shrink.
2. Within a group, the atomic radius grows from top to bottom. Electron shielding is to blame for this.
Metallic Character Trends
1. Over time, metallic qualities decrease from left to right. This is due to the atom's decreased radius (produced by Zeff, as previously explained), which permits the outer electrons to ionise more easily.
2. Metallic qualities become more prominent as you move down the group. Outside electrons in larger atoms ionize faster than electrons in smaller atoms as the atomic radius increases due to electron shielding.
3. The capacity to lose electrons is referred to as a metallic character, whereas the ability to receive electrons is referred to as a non-metallic character.
Also check-
Frequently Asked Questions (FAQs)
It is called the periodic table because of the way the elements are arranged. You'll notice they're in rows and columns. The horizontal rows (which go from left to right) are called 'periods' and the vertical columns (going from up to down) are called 'groups'.
Atomic number. The elements are listed in the Modern Periodic Table according to their atomic number rather than their relative atomic mass. The elements are grouped in the periodic table into rows, termed periods, in order of increasing atomic number. Vertical columns, referred to as groups, in which the elements share comparable characteristics of the Modern Periodic Table.
1. It's based on the atomic number of the elements.
2. The number of valence shells in each period is the same.
3. From left to right, the metallic characteristic decreases.
4. The number of valence electrons in a group is the same.
1. Hydrogen's position is unsatisfactory because its features in Modern Periodic Table are similar to those of both Group 1 and Group 17.
2. Isotopes do not have their own position.
3. Its primary body is unable to accommodate inner transition elements (Lanthanides and Actinides).
Ans: Groups are numbered one to eighteen. The s-block, or hydrogen block, of the periodic table contains two groups (1 and 2) of elements; the d-block, or transition block, contains ten groups (3 through 12); and the p-block, or main block, contains six groups (13 through 18).
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM

