Molarity And Mole Fraction: Definition, Formula, Questions
In chemistry, a solution is defined as a homogeneous mixture of two or more chemically non- reacting substances, the relative amounts of which can be varied upto a certain limit. If a solution consists of only two components, it is called a binary solution. The component present in smaller amount is called the solute while the other present in larger amount is called solvent.
This Story also Contains
- Solution:
- Concentration:
- Some Solved Examples
- Conclusion
The concentration of a solution can be expressed in the following ways: mass percentage or weight percentage, strength, molarity, molality, mole fraction, and normality. In this article, we will cover the concept of reactions in solutions. This topic falls under the broader category of some basic concepts of chemistry which is a crucial chapter in Class 11 chemistry.
Solution:
The solution is a homogeneous mixture of two or more chemically non-reacting substances whose composition can be varied within certain limits.
Solute and Solvent:
The solution is present in the same physical state as that of the solvent.
.jpg)
In case the species forming a solution are all present in the same physical state then the component that is present in a smaller amount is called the solute and the other present in a larger amount is called the solvent.
Concentration:
The concentration of a solution is a measure of the amount of solute that has been dissolved in a given amount of solvent or solution.
Types of Concentration Terms:
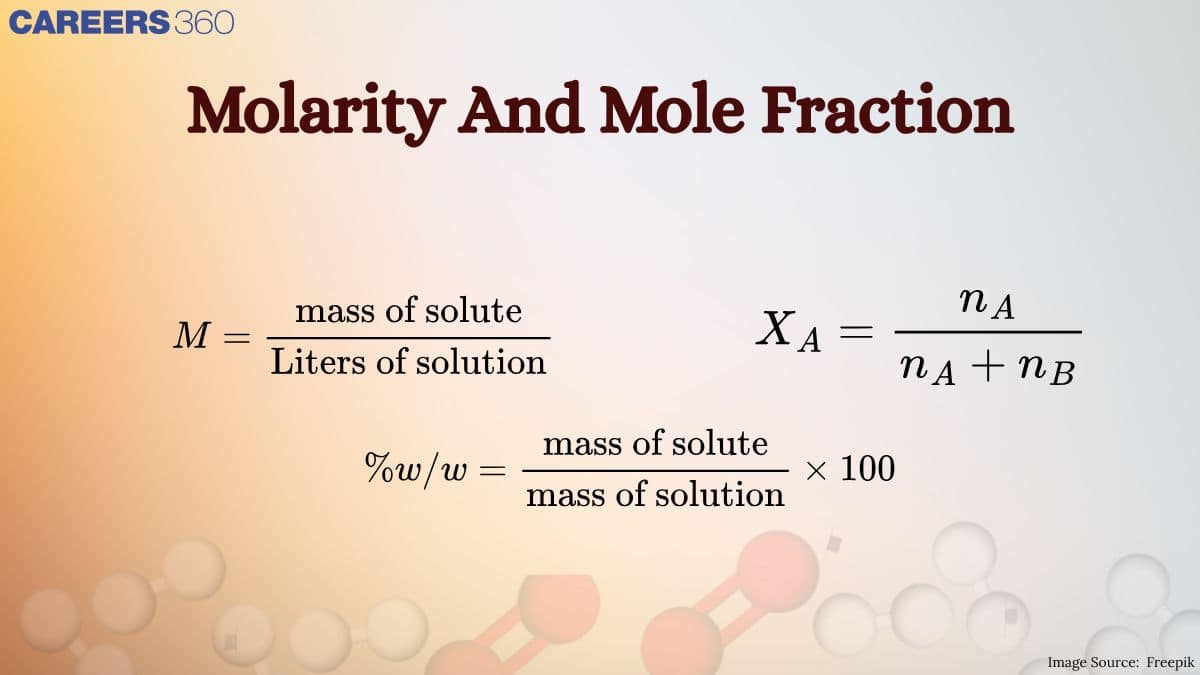
(I) Mass Percent or % (w/w)
Mass percent of a solute in a solution is the mass of the solute in grams present in 100g of the solution.
Mass Percent = (Mass of the component in the solution/ Total mass of the solution )×100
For example, if a solution is described as 10% glucose in water by mass, it means that 10 g of glucose is dissolved in 90 g of water resulting in a 100 g solution.
Example: A 10 mg effervescent tablet containing sodium bicarbonate and oxalic acid releases 0.25 ml of CO2 at T = 298.15 K and p = 1 bar. If the molar volume of CO2 is 25.9 L under such conditions, what is the percentage of sodium bicarbonate in each tablet?
[Molar mass of NaHCO3 = 84 g mol-1]
1) 0.84
2) 8.4
3) 16.8
4) 33.6
Solution:
2NaHCO3+H2C2O4→Na2C2O4+2CO2+2H2O
Here, number of moles of CO2=0.25×10−325.9≈10−5
Now, one mole of CO2 is produced by one mole of NaHCO3.
Thus,
The number of moles of NaHCO3 in the given reaction
= Number of moles of CO2=10−5
Hence,
% Mass =84×10−510×10−3×100=8.4%
Hence, the answer is (8.4%).
(II) Mole Fraction:
The commonly used symbol for mole fraction is x and the subscript used on the right-hand side of x denotes the component.
It is defined as:
Mole fraction = (Number of moles of the component /Total number of moles of all the components)
It is expressed by X for example, for a binary solution with two components A and B.
XA=(nA/nA+nB)
XB=(nB/nA+nB)
XA+XB=1
Here nA and nB represent moles of solvent and solute respectively. Mole fraction does not depend upon temperature as both solute and solvent are expressed by weight.
(III) Molality
It is defined as the number of moles of the solute dissolved in 1 Kg of the solvent.. It is denoted by 'm'.
m= Moles of thesolute / Mass of solvent in Kg
- If molality is one solution, it is called molal solution.
- One molal solution is less than one molar solution.
- Molality is preferred over molarity in experiments because it is a mass-based concentration unit that remains constant across temperature changes, while molarity, which is volume-based, varies with temperature due to the expansion or contraction of the solution's volume.
Example: Calculate the molality of a solution containing Acetic acid in Ethanol if the mass of solute = 10g and the density of Ethanol = 0.789 gmL-1.
1) 0.2112
2) 0.2012
3) 0.1992
4) 0.2002
Solution
We know.
Molality (m) = (number of moles of solute)/(mass of solvent in kg)
Now,
Moles of solute Acetic acid = mass / molar mass
=1060 moles
If Volume of ethanol = 1 L
Density = mass/volume
So,
Weight of ethanol = 1000 x 0.789 = 789g = 0.789 Kg
Molality = mass of solute mass of solution in kg
Molality =1060×7891000=0.2112 m/kg
Hence, the answer is (0.2112 m/kg).
(IV) Mass by Volume Percentage (w/v):
Another unit that is commonly used in medicine and pharmacy is mass by volume percentage. It is the mass of solute dissolved in 100 mL of the solution.
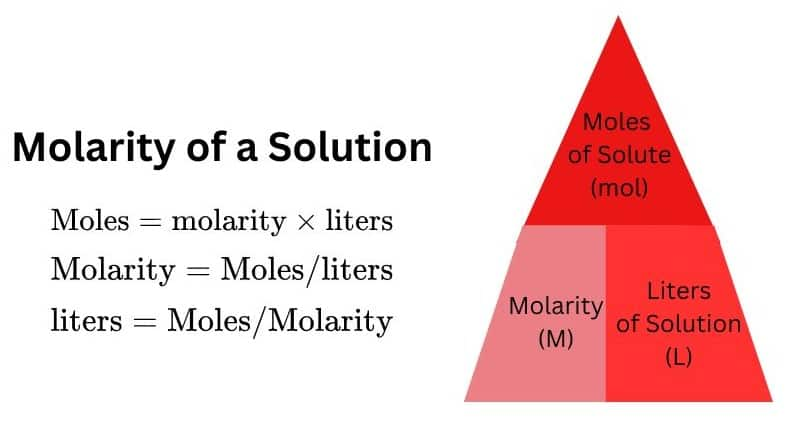
(V) Molarity:
It is the number of moles or gram moles of solute dissolved per liter of the solution. Molarity is denoted by 'M'.
M= (Moles of solute / volume of solution in litres or dm3)
- When the molarity of a solution is one, it is called a molar solution and when it is 0.1, the solution is called a decimolar solution.
- Molarity depends upon temperature and its unit is mol/liter.
- The number of moles of solute if the Molarity and the Volume in liters are given, is calculated as
Moles = M $\times$ V
In case the volume is given in ml then the millimoles of solute will be given by the above formula
- On dilution water is added and the final volume is made to V2 , then the moles of solute will remain constant and hence the following formula can be used (V1 is the volume before dilution, V2 is the volume after dilution)
M1V1 = M2V2
- When a mixture of different solutions having different concentrations is taken the molarity of the mixture is calculated as follows: M=M1V1+M2V2……
- When density and % by weight of a substance in a solution are given, molarity is found as follows:
M=(% by weight ×d×10)/( Molecular weight)
Here d = density
Example: What volume (in L) of solution of 2M BaSO4 contains 192 g of SO42- ion?
1) 0.5
2) 1
3) 2
4) 1.5
Solution
Molarity -
Molarity (M) = (Number of moles of solute)/(volume of solution in litres)
- wherein
It is defined as the number of moles of the solute in 1 liter of the solution.
192 g of SO42- = 2 moles of SO42-
2 moles of SO42- = 2 moles of BaSO4
Volume = no.of moles molarity =22=1L
Hence, the answer is an option (2).
(VI) Normality
It is the number of gram equivalents of solute present in one liter of the solution and it is denoted by 'N'.
N= (Mass of solute in gram /Equivalent mass of the solute)
- When the normality of a solution is one, the solution is called a normal solution and when it is 0.1, the solution is called a decinormal solution.
Normality Equation:
N1 V1=N2 V2
- Volume Of water added = V2 - V1
Here V2 = volume after dilution
V1 = volume before dilution - When density and % by weight of a substance in a solution are given, normality is found as follows:
N=(% by weight ×d×10 )/ (Equivalent weight)
Here d = density of solution
When a mixture of different solutions having different concentrations is taken the normality of the mixture is calculated as follows: N=N1V1+N2V2…
(VII) Strength :
It is the amount of solute in grams present in one liter of solution. It is denoted by C or S.
C or S=Mass of solute in gram /Volume in litre
C=N×E
Here N= normality and E= Eq. wt.
(VIII) The relation between Normality and Molarity :
N = molarity x n-factor
N x Eq wt. = molarity x molar mass
Also Read:
Recommended topic video on (Molarity And Mole Fraction )
Some Solved Examples
Example: A sample of KCl is placed in 50 ml of solvent. What should be the mass (in gm) of the sample for the molarity to be 2M ?
1) 7.45
2) 7.81
3) 6.81
4) 7
Solution
Number of Moles = molarity x volume
= 2 x 0.05 = 0.1
So, mass = (39 + 35.5) x 0.01g = 7.45 g
Hence, the answer is an option (1).
Example: The amount (in g) of sugar (C12H22O11) required to prepare 2L of its 0.1 M aqueous solution is
1) 17.1
2) 68.4
3) 136.8
4) 34.2
Solution
Molarity (M) = (Number of moles of solute)/(volume of solution in litres)
It is defined as the number of moles of the solute in 1 liter of the solution.
As we have learned in the mole concept.
Molarity (M) = (Number of moles of solute)/(volume of solution in litres)
Where,
No. of moles = (Weight of solute given/ Molar mass of solute)
0.1=wt/(342 x 2)
wt(C12H22O11) = 68.4 gram
Hence, the answer is the option (2).
Practice More Questions From the Link Given Below:
Conclusion
From the discussion of molarity and molality, it is evident that in molarity we consider the volume of the solution while in molality we consider the mass of the solvent. Therefore, the two are never equal. Molality is considered better for expressing the concentration as compared to molarity because the molarity changes with temperature because of the expansion or contraction of the liquid with temperature. However, molality does not change with temperature because the mass of the solvent does not change in temperature.