Monomers - Definition, Classification, Examples, FAQs
A monomer is a basic chemical compound for the formation of a large polymer chain by reacting together with another monomer molecule and the process involved is called polymerization. The polymer chain obtained is also called a three-dimensional network. The obtained molecule after the reaction and combination of monomer molecules is also called a macromolecule and we can say that a monomer molecule is the constitutional unit of the macromolecules.
This Story also Contains
- Classification of Monomers
- Classification based on the synthesis
- Condensation polymerization
- Addition polymerization
- Ring-opening polymerization
- Monomers and polymers
Monomers can also be defined as small molecules that are joined together with the help of a bond to form a larger molecule. Before going into the definition of monomer let us discuss the molecules there are mainly two types of molecules that may be macromolecules and micro molecules. The one which has small molecules is called micro molecules so monomers belong to micromolecules. While the molecules obtained after the combination of monomers are joined molecules or macromolecules.
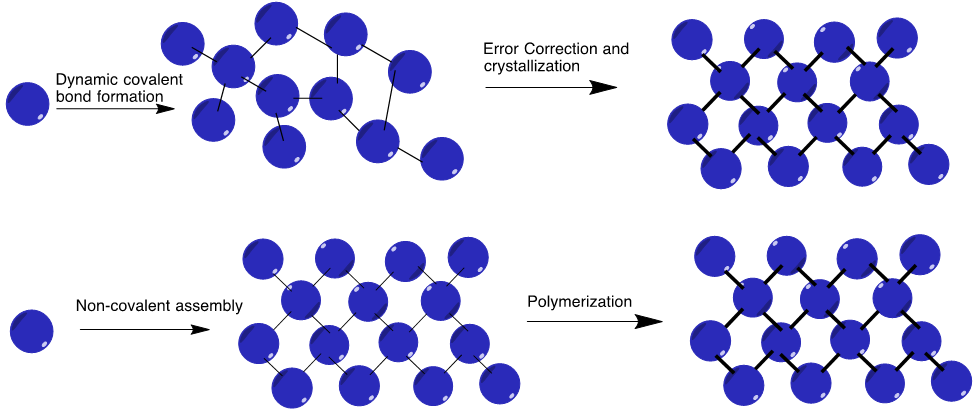
Polymerization of monomers
Also read -
Classification of Monomers
Monomers are chemical compounds consisting of atoms and molecules which then form a bond with each other and forms polymers or macromolecules. Natural and synthetic monomers are a very important classification of monomers.
Natural monomers
These types of monomers are found in nature and their organic molecules are combined together to form larger molecules or macromolecules. And these are responsible for the existence of life on our planet. They are biomolecules and are the building blocks of life on the earth. There are mainly five types of natural monomers, monosaccharides, amino acids, nucleotides, fatty acids, alcohols, and isoprene. Saccharides are natural monomers which then combined with form disaccharides and trisaccharides then polysaccharides.
The amino acids combined form the proteins. Amino acids are combined with peptide bonds. Nucleotides are building blocks of DNA and RNA that are the genetic material. The building blocks of lipids are alcohol and fatty acids. For natural rubber, the building block is isoprene that is isoprene monomers are combined together to form the natural rubber. So natural monomers are all present in the living organism naturally. Some of these important natural monomers can be explained in detail as follows.
Amino acids
The presence of amino and the acidic groups that is carboxylic group give the name amino acids. These are the building blocks of proteins. 10,000 proteins are formed with 20 amino acids. True proteins contain the elements carbon, hydrogen, Sulphur, and nitrogen. Glycine, glutamine, valine, arginine, and cysteine are examples of amino acids.
Nucleotides
Nucleotides are the monomer unit for DNA and RNA. For DNA the monomer is deoxynucleotides and for RNA the nucleotide is a ribonucleotide. And also polynucleotides consist of a nitrogen base that is pyrimidine and purine linked with the sugar-phosphate. And these are commonly found in the nucleus of the cell.
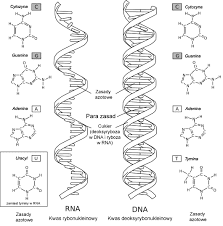
RNA and DNA
Monosaccharides
These are the simplest unit of carbohydrates. The monomer for carbohydrates is monosaccharides and contains glycosidic linkages. Glucose is the very important and most abundant monosaccharide. One of the very important monomer units is glucose and is the most abundant natural monomer found and is then combined with glycosidic bonds to form the polymers cellulose, starch, etc.
| Related topics link, |
Isoprene
The monomer unit of natural rubber is isoprene. Isoprene combined to form cis 1, 4 polyisoprene and trans 1, 4 polymers. Of which cis polyisoprene is commonly seen and important.

Natural rubber
Synthetic monomers
These are man-made or artificially found monomers that are then combined to form polymers that are useful for mankind. Its means that the building blocks that are the monomers are synthesized artificially with the help of different atoms and which then reacted together to form the molecules that can be used in various applications of human needs. For example, ethylene is a monomeric unit that is in the size and artificially for the production of polyethylene. tetrafluoroethylene which is a derivative of ethylene is prepared artificially for the production of a Teflon. Another important example of a synthetic monomer is caprolactam.
Also, students can refer,
Classification based on the synthesis
Based on the mode of synthesis of polymers from monomers they are adopted to form polymers they can be classified as one that participates in a condensation polymerization and the one that participates in addition polymerization.
Condensation polymerization
The monomer molecules that can lose small molecules as by-products like water, alcohol accepted in order to form a polymer is called condensation polymer. Some of the common condensation polymers obtained poly amides proteins, polyacetals. The monomers of condensation polymers are amide, etc.
Addition polymerization
This type of polymerization does not involve the generation of by-products like water, alcohol acceptors during their combination is addition polymers. It involves some simple molecules like alkenes, alkynes, etc. that are merely unsaturated compounds with the help of a free radical mechanism addition polymerization of them takes place. For example, the monomer olefin combined in an addition reaction for the formation of polyolefins.
This can also be initiated with the help of carbanions, metal complexes. PVC, polypropylene, Teflon, polystyrene, etc. are examples of additional polymers. The copolymer is also a type of addition polymer in which different types of monomers undergo an addition polymerization reaction for the formation of copolymers. One such example is the addition reaction of vinylidene chloride and vinyl chloride for the formation of Saran wrap.
Ring-opening polymerization
Some monomers that are of a ring or cyclic structure undergo polymerization for the formation of the ring-opening polymerization reaction. For example by the ring-opening reaction of ethylene oxide polyethylene glycol is formed.
Monomers and polymers
Monomers are micro molecules while polymers are macromolecules formed by the combination and reaction of monomer units. So polymers are chains consisting of many units of monomers. A single monomer unit can get combined with two other monomer units. Depending on the kind of monomer present polymers are classified as homopolymer and heteropolymers.
When monomer units of the same type are combined it is called homopolymer while different types of monomers when combined which result in the formation of heteropolymers. PVC, polystyrene, etc. are all polymers formed from monomers. Monomer and polymers are interconnected.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes: