Paper Chromatography - Principle, Examples, Types, Uses, FAQs
What is Paper Chromatography?
Paper chromatography is a chromatography technique that use paper sheets or strips as the adsorbent and stationary phase through which a solution is forced to flow. It is a low-cost way to separate dissolved chemical compounds based on their distinct migration speeds across sheets of paper. It is a highly effective analytical tool that requires very little material. In 1943, Synge and Martin invented paper chromatography. Paper chromatography is the example of partition chromatography.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Paper Chromatography?
- Principle of Paper Chromatography
- Instrumentation of Paper Chromatography
- Procedure of Paper Chromatography
- Paper Chromatography Diagram
- Types of Paper Chromatography
- Applications of Paper Chromatography
- Some Uses of Paper Chromatography
The stationary phase in this procedure is chromatography paper, which is suspended in a combination of solvents that serves as the mobile phase. We place the combination to be separated in an area at the bottom of the chromatographic paper, and as the solvent rises up the paper, the components are carried to varying degrees based on their retention on the paper. As a result, the components are separated at various heights.
Principle of Paper Chromatography
This technique is a type of partition chromatography in which the substances are distributed between two liquids, one of which is the stationary liquid (usually water) held in the paper fibres and referred to as the stationary phase, and the other of which is the moving liquid, also known as the developing solvent and referred to as the moving phase.
In paper chromatography, cellulose filter paper is frequently employed as the stationary phase.
It is generally covered in a thin coating of water because it is hydrophilic.
Liquid-liquid chromatography is a common term for the technique.
The divided components travel at different rates and emerge as spots on the paper at different times.
A drop of the test solution is put as a small spot on filter paper and dried in this process.
The filter paper is held in a small chamber and the edge is dipped into a solvent called developing solvent.
The various compounds are carried at different rates by the solvent system as soon as the liquid passes through the filter paper's capillary axis and reaches the spot of the test solution (a mixture of two or more substances).
The paper is dried and various areas are visible using a reagent called a visualising agent after the solvent has moved these substances to a proper height (15-18 cm).
Rf values are used to describe how compounds move in relation to the solvent (retardation factor or retention factor).
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Stationary Phase
It is liquid, that is, the water trapped in the molecular structure of the paper and is invisible.
Supporting material for the stationary phases is the matrix of cellulose fibres of chromatography paper.
Chromatography papers are available in three running characteristics: slow, medium, and fast.
Most frequently used chromatographic paper is Whatman No.1 or its equivalent.
Mobile Phase
The eluent is usually a liquid, such as a single solvent or a mixture of solvents that can travel through the paper in a pure paper system.
A water-organic mixture is commonly employed in mixed solvent systems, such as n-butanol, acetic acid: water (4:1:5, top layer) for flavonoid, glycosides, acetic acid: conc.HCl: water (30:3:10) (Forrestal system for flavonoid aglycones), toluene: acetic acid: water (for flavonoid aglycones), toluene (4:1:5, upper phase for flavonoid aglycones).
The solvents utilised are from the eluotropic series, which is a collection of solvents sorted in increasing polarity order.
In general, the greater a solute's solubility in a solvent, the greater its mobility in that solvent.
Because a solute dissolves more readily in the mobile phase, it will travel with the solvent, resulting in a partition between the two phases.
Because of their varying solubility in two phases, different solutes migrate at different rates up the paper.
Instrumentation of Paper Chromatography
It does not necessitate the purchase of pricey equipment.
The development chamber or tank is the only equipment required.
A closable container, such as a screw-capped glass bottle or jar, with a wire or clip attached to the lid to support the paper strip, is the simplest type.
There are two types of paper development devices: ascending and descending.
Procedure of Paper Chromatography
Step 1: Sample Preparation
The mixture to be analysed (for example, a mixture of amino acids) is dissolved in an appropriate solvent (0.5-3 %).
The solvent chosen should be volatile to allow for quick evaporation.
Step 2: Paper Selection
For analytical paper chromatography, Whatman No. 1 chromatography paper or an equivalent paper is employed.
For preparative paper chromatography, Whatman No.3 paper is used.
Step 3: Sample Loading or Spotting
For ascending paper chromatography, a pencil line is drawn across the paper 10-15cm from the bottom, and for descending paper chromatography, a pencil line is drawn 10-15cm below the anti siphon bar.
Using a capillary tube and a micropipette, a drop of solution is deposited on the paper at this line.
In the same way as the combination spot, reference compounds are created and applied to the paper.
This makes it easier to interpret the chromatogram.
Step 4: Development of Elution
The development of paper chromatograms can be done in a variety of development chambers or tanks.
A small jar or wide mouthed screw-capped bottles can be used to make small paper strips.
In a large rectangular glass tank, a large rectangular sheet of paper (20cm X 20cm) is created.
The solvent is poured into the tank so that the pencilled line is just visible above the solvent's surface.
The paper is removed from the tank when the solvent reaches practically the top of the paper, and the solvent is allowed to evaporate in the air until the paper is dry.
Step 5: Detection of Spot Location or Visualisation
If the separated components or solutes are coloured, they can be seen with the naked eye in daylight.
If the components are colourless, they must be revealed or located using a physical or chemical attribute.
Fluorescence and spray reagent are often used visualisation techniques, but because the paper is burned, conc. sulphuric acid is not suitable for paper chromatography.
Chemical reagents that react with functional groups to produce a colourful spot are known as spray reagents.
For example, following development, the paper is sprayed with a ninhydrin reagent solution and heated for 10 minutes at 110°C to produce a coloured product containing each of the amino acids.
Paper Chromatography Diagram
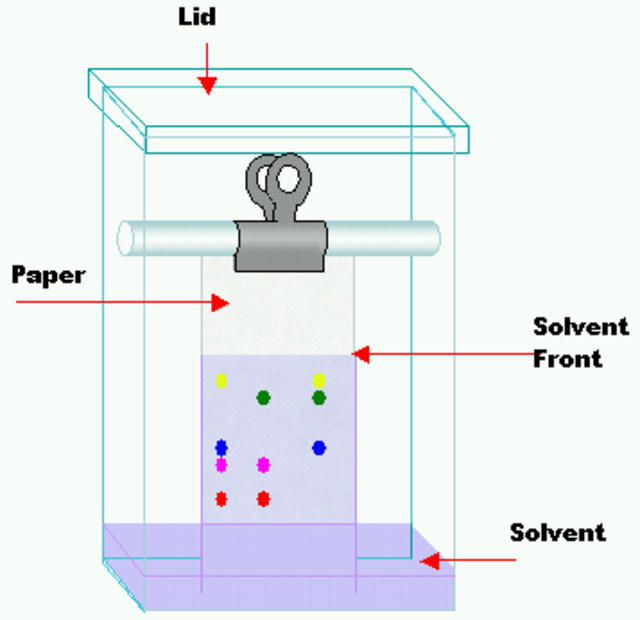
Related Topics Link, |
Types of Paper Chromatography
Ascending Paper Chromatography
The developing solvent is seen to be rising upward, as the name suggests. A suitable amount of mobile phase is poured into the development chamber at this point. On a line drawn a few centimetres from the bottom edge of the paper suspended from a hook or clip at the top, the sample and reference are spotted.
Descending Paper Chromatography
Inside the developing chamber, the solvent front goes down the length of paper suspended from the top. In the upper chamber, the mobile phase is held in a trough. The paper is clamped to the top with spots on a line marked a few centimetres from the top. The jar is covered and equilibrated with the mobile phase vapour before elution.
Ascending Descending Paper Chromatography
It is a combination of the two strategies mentioned above.
The upper section of ascending chromatography can be folded over a glass rod in this approach, allowing the ascending development to switch to descending after passing the glass rod.
Radial Paper Chromatography
Circular paper chromatography is another name for this technique.
- Radial development is used in this.
- A circular filter paper is used in this approach. Then, in the centre, the various materials to be studied are put.
- After the spot has dried, the paper is placed horizontally on the petri-dish containing the solvent, allowing the tongue or wick to dry.
- When the solvent front has travelled a sufficient length of time, the components divide into concentric circular zones.
Two Dimensional Chromatography
A square or rectangular piece of paper is used for this.
One of the corners receives the sample.
The second development is done at a right angle to the first run's direction.
This form of chromatography can be done with two solvent systems or with identical solvent systems in both directions
Also Read:
- NCERT solutions for Class 12 Chemistry Chapter 5 Surface Chemistry
- NCERT Exemplar Class 12 Chemistry Solutions Chapter 5 Surface Chemistry
- NCERT notes Class 12 Chemistry Chapter 5 Surface Chemistry
Applications of Paper Chromatography
Qualitative Analysis
It helps to determine which compounds are present in the mixture.
The usage of an Rf value based on the Rf of a standard compound is used to identify it.
Quantitative Analysis
It can be done while the component is still in the paper or after it has been removed.
The latter is usually preferred: the component is cut out of the paper, extracted with an appropriate solvent, and quantified with a colorimeter or UV-Vis spectrophotometer.
Alternatively, the extracted solution is vacuum evaporated to remove the solvent, and the resulting residue is weighed.
Preparative Paper Chromatography
Operates with large amounts (gram quantity) of substances to yield substances enough for further work in the laboratory.
Practically, it is done in Whatman No.3 paper. The sample is streaked.
The separated bands are cut, extracted with suitable solvent and filtered.
The filtrate is evaporated off in vacuum to yield the residue of the component.
Specific Applications
Separation of a variety of organic and biological compounds is included.
It has been used to determine indole in entire urine, as well as to examine barbiturates, antibiotics, hormones, and amino acids, among other things.
Some Uses of Paper Chromatography
To study the fermentation and ripening processes.
To ensure that medications are pure.
To examine cosmetics.
To identify pollutants in beverages and meals.
In biochemical laboratories, to examine reaction mixtures.
To determine the presence of drugs and dopes in humans and animals.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Chromatography is based on the idea of separating molecules in a mixture added to the ground, solid, or liquid stationary state (stable phase) while travelling with the help of a mobile phase.
In paper chromatography, Rf stands for retention factor, or the distance a fluid substance goes up a chromatography plate. All compounds have a common RF value for each solvent, and Rf values are used to match unknown samples to known compounds.
Paper Chromatography is an example of Partition Chromatography
Paper chromatography is used to detect adulteration in food and related industries.
It is also used to ensure the purity of medicinal products
Retardation factor(Retention factor) = Distance travelled by solute / distance travelled by the solution front
Also Read
19 Feb'25 04:59 PM
04 Nov'24 10:45 AM
07 Oct'24 12:46 PM
07 Oct'24 12:44 PM
04 Oct'24 06:04 PM
30 Sep'24 02:35 PM
30 Sep'24 02:28 PM
30 Sep'24 11:36 AM