Periodic Table Elements - Definition, Overview, Groups, FAQs
The elements of the periodic table, commonly called the "periodic table with atomic mass," are the representation of chemical elements in the increasing order of the atomic table or atomic number table, their chemical properties, and the order of their electronic configuration. The table comprises 118 total elements in periodic table. It is made up of 7 rows referred to as the periods and 18 columns referred to as the groups.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Why Was Classification of Elements Necessary?
- Law of Triads :
- Law of Octaves:
- Mendeleev’s Periodic Table:
- State The Modern Periodic Law And Modern Periodic Table:
- The Groups and Periods:
- Modern Periodic Table- Periods:
- Modern Periodic Table Groups:
- Periodic Trends:
- Ionization Energy:
- Electronegativity:
Why Was Classification of Elements Necessary?
As new periodic table elements were discovered, it became important to schedule and classify all of the existing elements. Physical and chemical properties, as well as atomic mass, were examined as a consequence. Various researchers tried to describe the elements based on chemical and physical characteristics.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Some of the accepted laws and methods were:
Law of Triads :
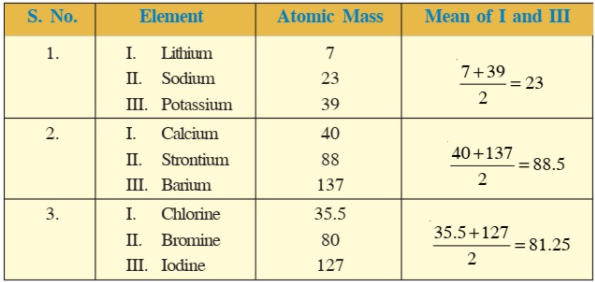
As per this law "If three elements are arranged in ascending order of their atomic masses that is atomic mass number of middle element is the Arithmetic mean of the first and third elements, then these elements will show similar properties".
This is known as "Law of Triads" as a group of three elements, having similar physical and chemical properties, repeated sequentially
Johann Wolfgang Döbereiner introduced this law in 1829.
For example, the mean atomic mass of lithium and potassium appeared closeby to the atomic mass of sodium.
Advantages: It identified that there is a relationship between properties of elements and their atomic masses.
Disadvantage: It was successful in identifying only three sets of triads from the then known elements. Also all elements did not exhibit this similarity.
Also read :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
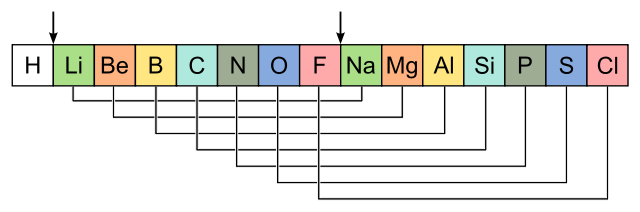
Law of Octaves:
This was established by John Newlands in 1966.
He identified that “every eighth element in the table had properties similar to that of the first''. He made a comparison to this with the octaves found in music.
He mentioned it as the ‘Law of Octaves’ also known as ‘Newland’s Law of Octaves’”.
Advantages: this law was acceptable only with lighter elements. This was the first periodical arrangement of elements. This was the first arrangement of elements in the increasing order of their atomic masses.
Disadvantages: invalid for elements lighter than calcium. Not applicable to all elements. Neglected the chance of identification of more elements in future.
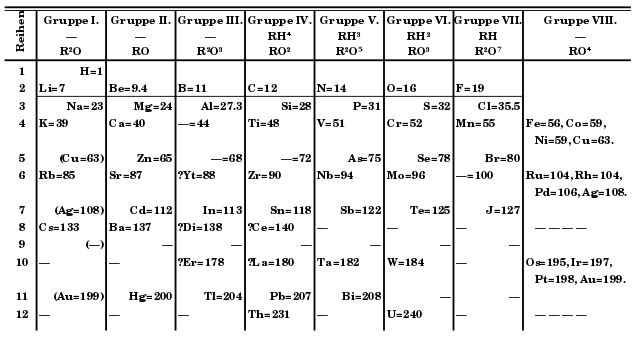
Mendeleev’s Periodic Table:
It consisted of 63 elements.
He focused on the relationship between an element's atomic mass of all elements and its physical and chemical properties. He noticed that the majority of the elements were assigned to a Periodic Table and organized in order of increasing atomic masses.
Advantage: Systematic study of elements Prediction of the properties of undiscovered elements. Position for Noble gases. Correction of atomic masses.
Disadvantages: He was not assigned a proper position. The position of isotopes was not successfully explained. Some elements were not arranged in their exact atomic mass order.
State The Modern Periodic Law And Modern Periodic Table:
Modern periodic table is given by Henry Moseley in 1913.
What is Modern Periodic Law? Modern Periodic Law is explained as: ‘Properties of elements are a periodic function of their atomic number.’ So, modern periodic table is based on atomic number.
The position of elements in the modern periodic table of elements speak about its chemical nature of reactivity.
How many groups and periods in periodic table?Full modern periodic table is made up of 7 rows referred to as the periods and 18 columns referred to as the groups.
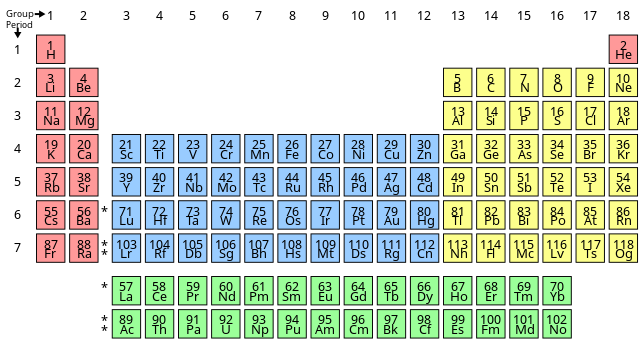
Modern periodic table with names is given above.
Advantages: modern periodic table given by moseley depends on a more fundamental property i.e. Atomic number It more clearly links the position of elements to their electronic configuration. It discussed the Periodic Table of Mendeleev's abnormalities and flaws. Since all isotopes of the same element have the same atomic number, they are now arranged in almost the same location. When these elements are ordered in order of increasing atomic mass, the paradox of higher atomic mass elements being placed before lower atomic mass elements (ie cobalt and nickel) is erased.
Related Topics link, |
The Groups and Periods:
Modern Periodic Table- Periods:
how many periods are there in modern periodic table? The periodic table of elements comprises 7 rows which are collectively called the period. Elements occupying the left hand end of the periodic table are metals while those elements occupying the right end are non metals. Borderline elements namely boron, silicon, germanium, arsenic have properties intermediate between those of the metals and non-metals. Hence they are collectively called metalloids or semimetals. The number of electron shells in an atom's electrical structure indicates its period. For example, if an element's atom contains two electron shells (K and L), it belongs to the second period.
Modern Periodic Table Groups:
How Many Groups Are There In Modern periodic table? The periodic table of elements is made up of 18 vertical columns that are collectively referred to as the groups. They carry elements with identical chemical behavior. 6 of the 18 groups are assigned specific names. For instance group 17 is called halogens, group 18 is referred to as noble gases. When chemical elements are grouped in the increasing order of atomic number, their properties follow a repeated pattern described as the "periodic law," in which elements in the same column group display similar properties. Elements having the same number of valence electrons occupy the same group. There is an increase in the number of shells as we proceed down the group marking new electronic shells getting occupied..
NCERT Chemistry Notes:
Periodic Trends:
Atomic radius :
GROUP TREND: As you progress through a group, the atomic radius grows. This is due to the filling of key energy state (1-7) with an increasing number of electrons. The ions are moving away from the center.
PERIOD TREND: As the atomic number grows, the radius of the atom shrinks from sequentially. This is because as the nuclei grows larger and more positive, greater electrons enter the same level. The electron cluster is drawn closer to the nucleus as a result of this.
Ionization Energy:
GROUP TREND: Ionization energy falls from largest to smallest in vertical groups. With this, the electrons are further away from the center, and the filled levels act as a shield.
PERIOD TREND: As you proceed from left to right toward the nobility, the ionisation energy tends to rise. This is due to the fact that metals lose electrons while nonmetals receive them.
Electronegativity:
GROUP TREND: Going top to bottom, electronegativity declines. Small atoms have a shorter distance between them. The proximity to the nucleus is greater, and the shielding effect is less.
PERIOD TREND: As you move across a period, the value of electronegativity rises. Metals desire to be emptied. So that they lose electrons in their subzones Nonmetals seek to gain electrons in order to become noble gases.
Also check-
Frequently Asked Questions (FAQs)
Hydrogen is first element in the periodic table.
Nuclear mass typically increases from top to bottom and decreases from left to right. Since the atomic number was created to serve as the foundation for classifying the elements on the periodic table, it will always rise from left to right and top to bottom.
Group 18 elements are called noble gases and have a completely filled electronic configuration.
Going top to bottom, electronegativity declines. Small atoms have a shorter distance between them.The proximity to the nucleus is greater, and the shielding effect is less.
Chemical elements in the increasing order atomic number of elements in the modern periodic table.
One of the fundamental ideas in chemistry is known as the periodic law. Each chemist, whether consciously or unconsciously, applies the Periodic Law when dealing with the chemical elements, their properties, and their chemical reactions. Periodic law was the primary force behind the creation of the modern periodic table.
Any two elements do not have the same valence shell electronic configuration at any given time. A periodic gradation from left to right exists for an element's physical properties, and as a result, different chemical properties change over time for different elements. Periodic property is the term used to describe this.
Periodic trends are regular patterns found in the periodic table that reveal information about an element's properties, including electronegativity, atomic radius, and ionising power. According to the periodic law, when elements are grouped by their atomic number, specific characteristics of those elements tend to repeat at regular intervals.
Also Read
12 Mar'25 09:34 AM
06 Feb'25 11:45 PM
06 Feb'25 11:36 PM
09 Jan'25 03:51 PM
16 Dec'24 11:23 PM
13 Nov'24 03:53 PM
23 Sep'24 01:11 PM