Periodicity of Valence or Oxidation States of Elements - Definition, Examples, Types, FAQs
Valency Definition: The valencies of all 118 elements of an atom refers to the number of electrons it gains or losses to complete its outermost shell. By completing their outer shell, or octet, atoms become more stable (8 electrons in the outermost shell). As a result, periodicity of valence or oxidation states of elements interacts with other atoms or participate in chemical reactions in which they can give or lose electrons to other atoms or take or acquire electrons from other atoms to develop a stable state.
This Story also Contains
- Valency Concept:
- What are valence electrons, and what do they do?
- How do you determine an element's valency?
- Oxidation Number vs Valency
- Types of Valency
- Electrovalence
- Covalency
- The Value of the First 30 Elements
As a result, they tend to interact with other atoms or participate in chemical reactions in which they give or lose electrons to other atoms or accept or gain electrons from other atoms to complete their octet and establish a stable state. So, the reason for bond formation or a chemical reaction between atoms is that atoms are always fighting to establish a stable state by clinging to each other. When an atom has one shell, for example, the stable state is obtained when it possesses two electrons. When an atom has two or more shells or orbits, the stable state is reached when the outermost shell possesses eight electrons. Hydrogen, for example, contains only one electron in the outermost or valence shell of its atom.
To complete electrons in its outermost orbit, it must add one more electron to its outermost shell or orbit. Similarly, the question arises what the valency of oxygen electronic arrangement of oxygen valence electrons is 2, 6. In its outermost shell or orbit, it has 6 electrons. As a result, it will require two additional electrons to complete the octet. Magnesium, with an atomic number of 12, has an electrical configuration of 2, 8, 2. To complete the octet, it will require 6 electrons. However, giving Mg 6 electrons is a difficult task for an atom. As a result, Magnesium's octet will be completed by donating or giving its two valence electrons to atoms of other elements.
Also read -
Valency Concept:
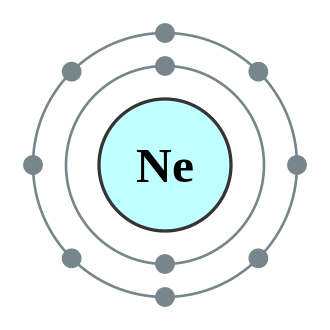
Noble gases are the least reactive because their periodicity of valence or oxidation states of elements shell is totally filled. The capacity of other elements to achieve the noble gas configuration determines their reactivity. For example: neon (Outermost shell is totally filled).
What are valence electrons, and what do they do?
The valence electrons are the total number of electrons present in an atom's outermost orbit or energy shell. Magnesium's electrical configuration, for example, is 2, 8, 2. It means that the K orbit contains two electrons, the L orbit has eight, and the M orbit has two. In Magnesium's outermost orbit, there are two electrons. As a result, it contains two valence electrons.
How do you determine an element's valency?
The loss or gain of electrons relates to valency, which is distinct from the total amount of electrons in an atom. Since, Sodium valency has an atomic number of 11, it contains 11 electrons but a valency of 1. It is easier for it to lose 1 electron than to add 7 electrons to reach stability of periodicity of valence or oxidation states of elements of complete its octet since its electronic distribution is (2, 8, 1).
As a result, its valency is 1. Similarly, the valency of oxygen and calcium valency with atomic number 8 and electronic configuration (2, 6) is 2 since gaining two electrons to complete its octet is easy for it. On the other hand, atoms having the electrical configuration (2, 7) tend to gain one electron rather than lose all their seven electrons since gaining one electron is easier than losing seven. As a result, its valency is 1.
There are also elements in the periodicity of valence or oxidation states of elements with zero valency, such as noble gases like helium (He), neon (Ne), and argon (Ar), because their outermost shell has 8 electrons, and their octet is complete. As a result, they do not react with other atoms or elements, or they are believed to be the least reactive, and they are therefore referred to as inert gases.
Furthermore, valency affects the charge on an atom when it forms an ion after losing or acquiring electrons. The charge on a sodium ion, for example, is -1 because it loses one electron in a process to achieve a perfect electronic configuration (2, 8) or a filled outermost shell or orbit. Similarly, when Magnesium loses two electrons, the charge of the ion is +2 (Mg2+). Because of its electrical structure (2, 8, 2), it has a tendency to lose two electrons.
NCERT Chemistry Notes:
Oxidation Number vs Valency
Both oxidation number and valency are expressions that refer to an atom's valence electrons. The main difference between the valency along with oxidation number is valence which in reference to the max-to-max number of electron which an atom can lose/gain/share fulfil the stability, meanwhile oxidation number is maximum number of electron which an atom can lose/gain for formation of the bond with another atom. The elements with valency 1 are diatomic molecules.
Types of Valency
Atoms unite to complete their octet, resulting in the production of compounds that can be either ionic or covalent. As a result, there are two forms of valency of all elements, as stated below.
Electrovalence
Electrovalence is a property of atoms that produce ionic or electrovalent compounds. The number of electrons lost or gained by an atom to establish a stable state or complete its octet is referred to as electrovalence. Positive ions (cations) are formed when an atom loses electrons, and their valency is called positive electrovalence. Negative ions (anions) are formed when atoms gain electrons, and their valency is called negative electrovalence.
So, electrovalence refers to the valency of ionic (electrovalent) compounds or electrovalent compounds that are generated when metals and non-metals come together. For example, sodium chloride (NaCl) is a non-metal with a metal component. The transfer of electrons from Sodium to Chlorine forms the chemical link between the atoms of sodium and chlorine in the Sodium Chloride molecule. Chlorine gains one electron whereas sodium loses one. As illustrated below, the electrovalence of sodium and chlorine is 1 because Na loses one electron and Cl gains one.
Na++Cl-→NaCl
Also read :
- NCERT notes Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT solutions for Class 11 Chemistry Chapter 3 Classification of Elements and Periodicity in Properties
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 3 Classification of Elements and Periodicity in Properties
Covalency
Covalency Definition: The number of electrons shared by atoms or elements during the formation of a covalent molecule is referred to as the number of electrons shared by the atoms or elements. The chemical link between non-metals is established in covalent compounds for periodicity of valence or oxidation states of elements. Covalent compounds are formed when atoms of one non-metal unite with atoms of other non-metals to create molecules. The atoms do neither gain nor lose electrons in this situation; instead, they share electrons. In Methane (CH4), for example, one carbon atom, which requires four electrons to complete its octet, unites with four hydrogen atoms, each of which requires one electron to complete the two electrons necessary in its initial orbit.
The Value of the First 30 Elements
Consider the valency chart or valency of the first 30 elements in the periodic table with valency.
VALENCY TABLE | ||
ELEMENT NAME | VALENCY OF ELEMENTS | ELEMENT’S ATOMIC NUMBER |
VALENCY OF HYDROGEN | 1 | 1 |
VALENCY OF HELIUM | 0 | 2 |
VALENCY OF LITHIUM | 1 | 3 |
VALENCY OF BERRYLLIUM | 2 | 4 |
VALENCY OF BORON | 3 | 5 |
VALENCY OF CARBON | 4 | 6 |
VALENCY OF NITROGEN | 3 | 7 |
VALENCY OF OXYGEN | 2 | 8 |
VALENCY OF FLORINE | 1 | 9 |
VALENCY OF NEON | 0 | 10 |
VALENCY OF SODIUM | 1 | 11 |
VALENCY OF MAGNESIUM | 2 | 12 |
VALENCY OF ALUMINIUM | 3 | 13 |
VALENCY OF SILICON | 4 | 14 |
VALENCY OF PHOSPHOROUS | 3 | 15 |
VALENCY OF SULPHUR | 2 | 16 |
VALENCY OF CHLORINE | 1 | 17 |
VALENCY OF ARGON | 0 | 18 |
VALENCY OF POTASSIUM | 1 | 19 |
VALENCY OF CALCIUM | 2 | 20 |
VALENCY OF SCANDIUM | 3 | 21 |
VALENCY OF TITANIUM | 4 | 22 |
VALENCY OF VANADIUM | 5,4 | 23 |
VALENCY OF CHROMIUM | 2 | 24 |
VALENCY OF MANGANESE | 7,4,2 | 25 |
VALENCY OF IRON | 2,3 | 26 |
VALENCY OF COBALT | 3,2 | 27 |
VALENCY OF NICKEL | 2 | 28 |
VALENCY OF COOPER | 2,1 | 29 |
VALENCY OF ZINC | 2 | 30 |
The valency of first 20 elements is given in above table.
Also check-
