Phenyl Group - Preparation, Structure, Properties, Occurrence, Uses, FAQs
Phenyl group is generally referred to as aryl phenols whereas aryl refers to compounds which contain aromatic rings along with phenolic group. Basically, If any functional group contains an aromatic group then the aryl group is represented as Ar. Phenyl group is formed when one of the hydrogen atoms of benzene is removed. Then the phenyl group is attached with this atom or any other atoms.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Phenyl Structure
- History of Word Phenyl
- Phenyl Structure, Bonding and Characterization
- Preparation of Phenyl Group
- Chemical Properties of Phenyl
- Occurrence of Phenyl Group
- Phenyl Uses
- Types of Phenyl:
Those aromatic phenyl groups which are present in the shape bridging polyene helps to improve the thermo and photochemical stabilities of chromophores. In simple language the phenyl group is said to be a cyclic group of atoms. The phenyl chemical or phenyl molecular formula is![]() whereas aryl group formula is
whereas aryl group formula is ![]() . These are closely related to benzene only one hydrogen atom is more in benzene chemical formula represented by benzene is
. These are closely related to benzene only one hydrogen atom is more in benzene chemical formula represented by benzene is![]() .
.
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
Phenyl Structure
Generally phenyl group contains six carbon atoms or we can say that phenyl composition consists of six carbon atoms which further bonded together in a shape of hexagon which forms planar ring out of which five are bonded with individual hydrogen atoms and the remaining one carbon bonds to any substituent group. In organic chemistry phenyl groups are commonly known. The structure of phenyl group can be shown as follows:
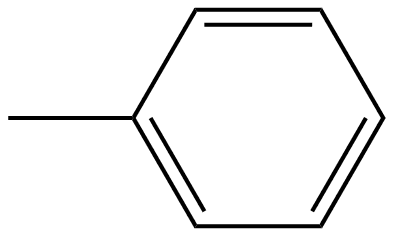
We can see that the alternative double and single bonds are there in phenyl groups like benzene and these are said to be aromatic in nature and it contains equal bond lengths between carbon atoms in the ring.
Phenyl is also known by the name phenyl radical and 3-D structure of phenyl can also be shown as follows:
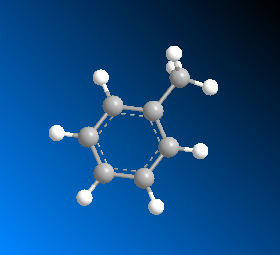
Phenyl appears as white crystalline solid which has a phenylic smell and it is poorly soluble in water. The symbol Ph is generally known for a Phenyl group. It is generally combined with hydrogen or other atoms of groups. When any of the group attached with phenyl like ![]() then it is named as chlorophenyl. This represents that phenyl group is mentioned in the last name while any of the substituents will be mentioned at first position.
then it is named as chlorophenyl. This represents that phenyl group is mentioned in the last name while any of the substituents will be mentioned at first position.
History of Word Phenyl
Phenyl meaning is derived from the French word phenyle which is further derived from Greek word phaino which means shining this name is chosen to give them as first few phenyl compounds discovered by making and refining various gases for lightning purposes. According to the concept given by McMurry the Pheno word represents the meaning “I bear light’’.
Phenyl Structure, Bonding and Characterization
As already discussed, the phenyl group is derived from benzene but the difference in phenyl and benzene can be demonstrated easily in terms of electronic properties. The phenyl group can relate with the vinyl group. Whereas vinyl is the functional group represented by the chemical formula ![]() which is ethylene. It is considered that conjugation is possible due to the presence of inductively withdrawing group due to higher electronegativity of
which is ethylene. It is considered that conjugation is possible due to the presence of inductively withdrawing group due to higher electronegativity of ![]() carbon atoms and presence of resonance donating group known as +M effect have ability to donate
carbon atoms and presence of resonance donating group known as +M effect have ability to donate ![]() electrons.
electrons.
This phenyl group is hydrophobic in nature whereas hydrophobic is that physical property of molecules by which it can easily attract towards water. These are known as non-polar in nature. Phenyl group is restricted towards oxidation and reduction. Like all aromatic compounds, phenyl group is also highly stable in nature and this stability is due to the unique properties of aromatic orbitals. Bond lengths of carbon atoms between phenyl groups is measured to be of ![]() . If we examine phenyl groups in NMR spectroscopy then it shows chemical shift at 7.27 ppm and these shifts are due to the presence of aromatic ring and also varies by the substituents attached to the ring.
. If we examine phenyl groups in NMR spectroscopy then it shows chemical shift at 7.27 ppm and these shifts are due to the presence of aromatic ring and also varies by the substituents attached to the ring.
Preparation of Phenyl Group
Phenyl groups are generally used as reagents which can behave as a source of phenyl anion or cation. The main reagents of phenyl are phenyl lithium which is represented by the chemical formula ![]() and phenyl magnesium bromide represented by
and phenyl magnesium bromide represented by![]() . These are said to be phenyl structural formulas.
. These are said to be phenyl structural formulas.
Phenyl are generally prepared by the attack of electrophiles on benzene atom and the reaction can be shown as:
![]()
Here ![]() is electrophile where electrophile are those compounds which are made up from two words called electro and phile where electro means electron and phile means loving i.e. those species that are electron loving in nature. Here the
is electrophile where electrophile are those compounds which are made up from two words called electro and phile where electro means electron and phile means loving i.e. those species that are electron loving in nature. Here the ![]() may be
may be ![]() and these types of reactions are called electrophilic substitution reactions. These reactions are generally organic reactions in which an electrophile replaces an atom which is attached to an aromatic ring. These reactions generally forward by the replacement of a hydrogen atom which belongs to a benzene ring with the help of an electrophile.
and these types of reactions are called electrophilic substitution reactions. These reactions are generally organic reactions in which an electrophile replaces an atom which is attached to an aromatic ring. These reactions generally forward by the replacement of a hydrogen atom which belongs to a benzene ring with the help of an electrophile.
Chemical Properties of Phenyl
One of the main known reactions of phenyl is in the form phenol which reacts with bromine solution which forms bromo substituted phenol along with hydrogen bromide. Reaction can be shown as follows:
![]()
Also Read:
- NCERT solutions for Class 11 Chemistry Chapter 13 Hydrocarbons
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 13 Hydrocarbons
- NCERT notes Class 11 Chemistry Chapter 13 Hydrocarbons
Occurrence of Phenyl Group
In many natural as well as synthetic organic compounds phenyl groups are present. One of the main natural product of phenyl is amino acid phenylalanine which contains a group of phenyls rather than natural one there is a big petrochemical industry product known by the name “BTX” which consists of benzene, toluene, and xylene these all are known as building blocks of phenyl compounds.
Phenyl Uses
Phenyls have many industrial and chemical uses which can described as below:
1. It is used in various institutional and domestic disinfectants when used in the combination of some other phenolic compounds. Like in homes we use phenyl to clean floors and toilets it also kills germs and acts as disinfectant.
2. Phenyl also has some odor so it can be used for removing bad odor.
3. Phenyl is one of the main disinfectants which is used in schools, offices, hotels and stores etc.
4. Phenyls also contain pharmacological properties due to which it can be used as antioxidants, analgesics etc.
Types of Phenyl:
There are many types of phenyl which can be expressed as follows:
1. Green phenyl: This is that type of phenyl which is generally used for removing insects from homes and as the name suggests green phenyl it is eco-friendly and non-toxic in nature.
2. Black phenyl: Black phenyl will act as a strong disinfectant which is black or slightly brown in color.
3. White phenyl: As the name suggests this phenyl is white or milky in color which is generally used to remove odors and for killing bacteria in homes, offices etc. Generally at homes we use white phenyl.
Phenyl is also said to be economically favorable as it comes in the range of 40-2500Rs according to their quality.
Also check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
Frequently Asked Questions (FAQs)
Phenyl and phenol are not the same. The main difference between the two terms is that phenol compounds have an oxygen atom in it while phenyl does not consist of any oxygen atom. Phenyl is a benzene ring in which one hydrogen atom is less as compared to benzene while phenol is an aromatic compound having an alcoholic group.
No, the given statement is not true as phenyl groups resist oxidation and reduction as they have high stability as compared to aliphatic groups.
These are generally cyclic groups which are closely related to benzene and can be defined as that benzene which has one lesser atom of hydrogen that atom will be substituted by any other compound.
These are generally cyclic groups which are closely related to benzene and can be defined as that benzene which has one lesser atom of hydrogen that atom will be substituted by any other compound.
These are generally cyclic groups which are closely related to benzene and can be defined as that benzene which has one lesser atom of hydrogen that atom will be substituted by any other compound.
These are generally cyclic groups which are closely related to benzene and can be defined as that benzene which has one lesser atom of hydrogen that atom will be substituted by any other compound.
Also Read
11 Mar'25 06:34 PM
11 Mar'25 06:27 PM
11 Mar'25 06:25 PM
11 Mar'25 05:54 PM
11 Mar'25 05:27 PM
05 Mar'25 05:23 PM
05 Mar'25 05:18 PM
05 Mar'25 04:59 PM
19 Feb'25 07:36 PM
19 Feb'25 07:33 PM