Pi Bonds: Definition, Formula, Examples, Questions
Pi bonds arise as a result of the lateral overlap of the p orbitals. Unsaturated hydrocarbons, having double and triple bonds, contain pi bonds. These are found in alkenes and alkynes. Such pi bonds confer unusual properties and reactivities on the compounds and hence modify their behavior in a chemical reaction. For example, the presence of pi bonds allows alkenes to undergo electrophilic addition reactions.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Understanding Pi Bonds
- Kinds of Pi Bonding: P-P and P-D
- p (pi-pi) Bonding
- Pi Bonds: Real-Life Applications
- Some Solved Examples
- Summary
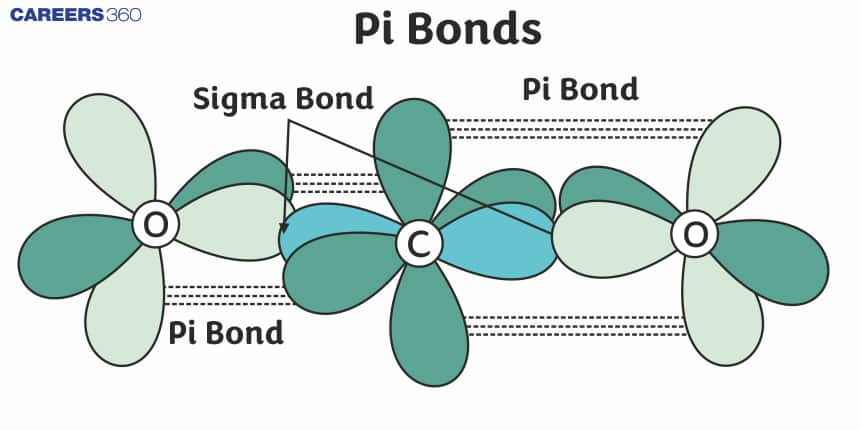
Understanding Pi Bonds
The pi bond is a covalent bond where the overlap between atomic orbitals is lateral, especially relating to p orbitals. This occurs when lobes of one p orbital align with those of another, resulting in a bond fundamentally different from the stronger sigma bond. Pi bonds have electron density above and below the plane of the nuclei of the bonding atoms, hence they are, in general, comparatively weaker than sigma bonds. One sigma and one pi bond is, structurally, a typical double bond and a triple bond consists of one sigma bond and two pi bonds.
Understanding pi bonding is important in molecular geometry because their existence restrains the rotational movement due to the fixed orientation of p orbitals on the axis of the bond. This becomes very vital in unsaturated compounds wherein the pi bonds result in specific chemical reactivity and properties.
Kinds of Pi Bonding: P-P and P-D
The major types of pi bonding include pi (p-p) and pi (p-d) bonding.
Pi (p-p) Bonding
Pi (pp) bonding occurs between the two 'p' orbitals of the adjacent atoms. This is so in ethylene, C₂H₄.
The carbon atoms in this molecule are sp² hybridized with one unhybridized p orbital on each carbon atom. These p orbitals overlap laterally and form the pi bond—which is above and below the plane of the molecule—to a large degree. This kind of bonding is frequent in alkenes and generally accounts for their typical reactivity, for instance, in electrophilic addition.
Pi (p-d) Bonding
The other is pi (p-d) bonding, where p orbitals are mixed with d orbitals, which is usually observed in transition metals. This kind of bonding is quite important for the creation of metal-metal bonds in compounds like metal carbonyls and metal complexes. Pi (p-d) bonds can exert a strong influence on the electronic properties of these complexes, which means their stability and reactivity.
The two kinds of pi bonding serve to illustrate just how versatile these bonds can be in different chemical settings and, therefore, their importance both in organic chemistry and inorganic.
p (pi-pi) Bonding
When one p-orbital of one atom contains one electron and the other atom has one electron in the p orbital, both perpendicular to the plane of the molecule, the type of bond formed is known as pπ−pπ bonding.
When one p-orbital of one atom contains one electron and the other atom has one electron, the type of bond formed is known as pπ−pπ bonding. In other words, pπ−dπ bond is formed when the p orbital of one atom and the d orbital of another atom overlap laterally.
For example, PO43- has a pπ−dπ bonding because the lateral overlap of the p-orbital of an oxygen atom with the d-orbital of a sulfur atom occurs.
Pi Bonds: Real-Life Applications
Pi bonds have several applications that stretch beyond theoretical chemistry into real life.
Pi bonds are central in organic chemistry to not only the structure but also the reactivity of unsaturated hydrocarbons. Take alkenes or alkynes as examples; these are unsaturated hydrocarbons with at least one or more pi bonds. They form very essential intermediates for many synthetic reactions. The pi bond can undergo electrophilic addition that empowers the chemist to take simple molecules and then transform them into complex compounds. Hence, they become very important in the synthesis of drugs or even materials science.
Materials Science
Pi bonds are important in determining the properties of various polymers. For instance, under conjugated systems where there are alternating single and double bonds, special electronic properties realize that are utilized in organic electronics and photovoltaic devices. In such materials, pi bonds allow the delocalization of electrons, hence enhancing conductivity and light absorption.
Biochemistry
The functions associated with π bonds within the structure of biomolecules are of immense importance in biochemistry. For instance, double bonds in fatty acids and the aromatic rings of nucleotides are of fundamental importance for the functions associated with the respective lipids and nucleic acids. Thus, the physical properties of such molecules result from the presence of π bonds that modulate their interactions and biological activities.
Recommended topic video on (Pi Bonds )
Some Solved Examples
Example 1
Question: What is the number and type of bonds in C22- in the CaC2?
1) one sigma 2 pi
2) two sigma one pi
3) one sigma one pi
4) 2 sigma 2 pi
Solution: The structure of C22- is (C-= C). In this ion, there is one sigma bond and two pi bonds. There fore correct option is 1.
Example 2
Question: Which one of the following does not have a pyramidal shape?
a) (SiH3)3 N
b) (CH3)3N
c)(SiH3)3
d) (CH3)3
Solution: The molecule (SiH3)3 N has a trigonal planar shape due to sp2 hybridization, while(CH3)3N does have a pyramidal shape. Thus, the correct answer is option (1)(SiH3)3 N
Example 3
Question: How strong is the ppi-ppi bond compared to ppi-dpi and dpi-dpi bonds?
1) More stronger than ppi -dpi and dpi-dpi bond
2) Less strong than ppi-dpi and dpi-dpi bond
3) More stronger than ppi-ppi and dpi-dpi bond
4) none of the above
Solution: The ppi-ppi bond is stronger than both ppi-dpi and dpi-dpi bonds. Therefore, the correct answer is option (1) More stronger than ppi-dpi and dpi-dpi bond.
Example 4
Question: How many ppi-ppi and ppi-dpi bonds are present in SO3?
1) 2, 2
2) 2, 1
3) 1, 2
4) 1, 0
Solution: In the structure of SO3, there is 1 ppi-ppi bond and 2 ppi-dpi bonds. Thus, the correct answer is option (3) 1, 2.
Example 5
Question: What is the number of ppi-ppi and ppi-dpi bonds respectively in P4O10?
1) 2,1
2) 1,2
3) 1, 4
4) 0, 4
Solution: In P4O10, all four π bonds (P=O) are ppi-dpi bonds, as phosphorus is sp3 hybridized. Therefore, the correct answer is option (4): 0, 4.
Summary
The paper treated pi bonds, focusing on their definitions, types, and applications in real life. Pi bonds form as a result of a lateral overlap of p orbitals. Therefore, it is of prime importance in the explanation of molecular structure and reactivity. There were two notable types of bonding: pi, or p-p, found in alkenes and alkynes, and pi, or p-d, bonding seen in transition metal complexes.
Also Read
19 Feb'25 11:09 AM
07 Feb'25 10:38 AM
07 Feb'25 10:22 AM
07 Feb'25 10:18 AM
07 Feb'25 10:16 AM
07 Feb'25 10:11 AM
18 Oct'24 05:56 PM
18 Oct'24 10:52 AM
10 Oct'24 12:05 AM
09 Oct'24 11:41 PM

