Preparation & Purification Of Colloidal Solutions
Sipping milk, smearing your favorite lotion, or even just looking out the window on a misty morning—each of these experiences has one thing in common—colloidal solutions. The functions that colloids play are of extremely core nature in many everyday products or even in industrial applications; now, knowing how such colloidal solutions are prepared and purified may throw more light onto their significance and efficiency for a myriad of applications.
This Story also Contains
- Colloids
- Peptization (Physical Method of Preparation)
- Chemical Methods of Preparation
- Properties of Colloidal Solutions
- Coagulation/Flocculation
- Electric Double Layer - Zeta Potential
- Sols for Protection - Gold Number
- Purification/Precipitation of Colloids
- Applications and Relevance
- Summary
Colloids
Colloids are mixtures in which one substance is dispersed evenly throughout another. The dispersed particles become larger than the molecules but small enough to remain suspended and not settle. In colloids, this may occur in any form—solids, liquids, or gases. Examples include milk, wherein it occurs from liquid in liquid; fog, wherein it occurs from liquid in gas—or jelly, wherein it takes place from solid in liquid.
Peptization (Physical Method of Preparation)
Peptization is the process in which a precipitate is converted into a colloidal solution by the addition of a small amount of an electrolyte. This method is followed when the precipitate has already been formed and on the addition of an electrolyte, the particles disperse nicely and uniformly.
Chemical Methods of Preparation
Chemical methods include the chemical reactions that yield colloidal particles. Some of the common techniques in use are processes of reduction, oxidation, hydrolysis, and double decomposition. For example, when Silver nitrate is reduced using tannic acid, the colloidal solution of silver is obtained.
Properties of Colloidal Solutions
Electrophoresis
Electrophoresis is the process by which colloidal particles move under the influence of an applied electric field. This technique allows for the determination of colloidal particle charge and is, for example, of great help in analytical techniques like DNA analysis or separation of proteins.
Coagulation/Flocculation
Coagulation or flocculation is the process of aggregation of colloidal particles into bigger particles causing their eventual precipitation. This process is important in water purification and in the treatment of wastewater.
Charge on Colloids
Hence, colloidal particles bear an electric charge, preventing aggregation due to mutual repulsion. The charges can be either positive or negative, based on the nature of the particles and dispersion medium.
Electric Double Layer - Zeta Potential
The electric double layer refers to the ions surrounding a colloidal particle. The potential difference between the surface of the particle and that between the dispersion medium remains known as zeta potential. It measures the stability of colloidal solutions.
Hardy-Schulze Rule
According to the Hardy-Schulze rule, the higher the charge of the ion, the higher will be its coagulating power. Multivalent ions exhibit better power of coagulation than monovalent ions.
Sols for Protection - Gold Number
Protective solids are those colloids that protect the coagulation of other colloids. The gold number expresses the protective power of a sol. It is the minimum amount of protective sol that when added to a given amount of gold sol, prevents its coagulation.
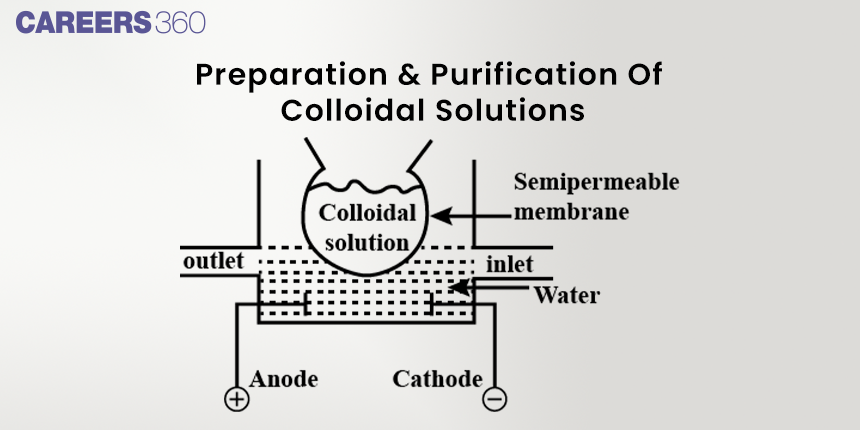
Purification/Precipitation of Colloids
Resalement procedures include dialysis, ultrafiltration, and centrifugation. The methods remove impurities and extra electrolytes in the colloidal solution to make it stable, that is, suitable for its purpose.
Applications and Relevance
Colloids have an important place in industries and research sectors. For example, in food industries, such as mayonnaise and salad dressings, a good texture and more shelf life will be provided by colloids along with emulsion stabilization. Moreover, in the case of ice cream, pharmaceuticals with drug delivery systems are colloidal, which helps to enhance the solubility and therefore the bioavailability of the variants of the drugs, hence resulting in better treatment.
Environmental science utilizes colloids in techniques such as water purification. Coagulation and flocculation methods eliminate colloidal particles from drinking water. Most of the colloids find an extensive application in the preparation of nanomaterials that have some fascinating properties with a wide application domain in electronics, medicine, and material science.
Finally, colloid science applies to chemistry, physics, and biology. The understanding of colloidal interactions underlies the development of new materials and an understanding of biological phenomena that drive innovation in both nanotechnology and medicine. Interdisciplinarity lends a plus to colloid science by facilitating collaboration and fueling successive waves of scientific and industrial innovation.
Recommendedtopicvideo on(Preparation & Purification Of Colloidal Solutions)
Some Solved Examples
Example 1
Question:
Colloidal gold is prepared by:
1) Mechanical dispersion
2) Peptisation
3) Bredig's Arc Method (correct)
4) Hydrolysis
Solution:
Colloidal sols of metals such as gold can be prepared using Bredig's Arc Method. This process involves dispersion and condensation, where intense heat produced by an electric arc vaporizes the metal, which then condenses to form colloids.
Hence, the answer is option (3).
Example 2
Question:
Peptization is a:
1) process of bringing colloidal molecules into solution
2) process of converting soluble particles to form a colloidal solution
3) process of converting precipitate into colloidal solution (correct)
4) process of converting a colloidal solution into a precipitate
Solution:
Peptization is the process of converting a precipitate into a colloidal solution. This involves the absorption of ions from an electrolyte on the surface of the precipitate, causing it to disperse into colloidal particles.
Hence, the answer is option (3).
Example 3
Question:
The crushing or grinding of ore is done by:
1) Peptization
2) Colloidal mill (correct)
3) Bredig's arc method
4) Double decomposition method
Solution:
By the dispersion method, using a colloidal mill, larger-sized particles are broken down into colloidal-sized particles.
Hence, the answer is option (2).
Summary
Colloids have a very vital role in our normal daily life and in scientific research applications. The areas of applications range from food production farms to pharmaceutical industries and the environment. In this paper, we consider the colloidal solution preparation and purification, methods of physical and chemical techniques, properties of electrophoresis, and coagulation along with concepts such as electric double layers and Hardy-Schulze rule. We also view their relevance and applications to industries and academic fields.
A greater understanding of colloids CFO points to their contribution to many products and processes. Continuous R&D on colloid science engenders technological advancement toward new solutions that touch everyday life and industries.
Frequently Asked Questions (FAQs)
The Hardy-Schulze rule states that the greater the charge, the greater the coagulating power of the ion. Thus, multivalent ions are far more effective than monovalent ions in causing coagulation.
Methods that may be used to purify them include dialysis, ultrafiltration, and differential centrifugation. These remove the impurities and excess amounts of electrolytes from the solution and thus help in maintaining the stability and effectiveness of colloidal solutions.
Peptization is a physical method for the preparation of colloids whereby, upon the addition of a small amount of any electrolyte, a precipitate is turned into a colloidal solution.
Chemical methods involve reduction, oxidation, hydrolysis, and double decomposition to obtain colloidal particles. For example, reduction of silver nitrate by tannic acid gives a colloidal solution of silver.
Electrophoresis is the migration of colloidal particles under an electric field; this can be used for determining the charge on particles and is utilized for DNA analysis and separation of proteins.
