Relation between Kp and Kc
The equilibrium constants ( Kp ) and ( Kc ) are fundamental concepts in chemical equilibrium, describing the relationship between the concentrations or partial pressures of reactants and products in a chemical reaction at equilibrium. In chemical thermodynamics, the equilibrium constants ( Kp ) and ( Kc ) provide crucial information about the position of equilibrium for a reaction.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Relation Between Kp And Kc
- Some Solved Examples
- Summary
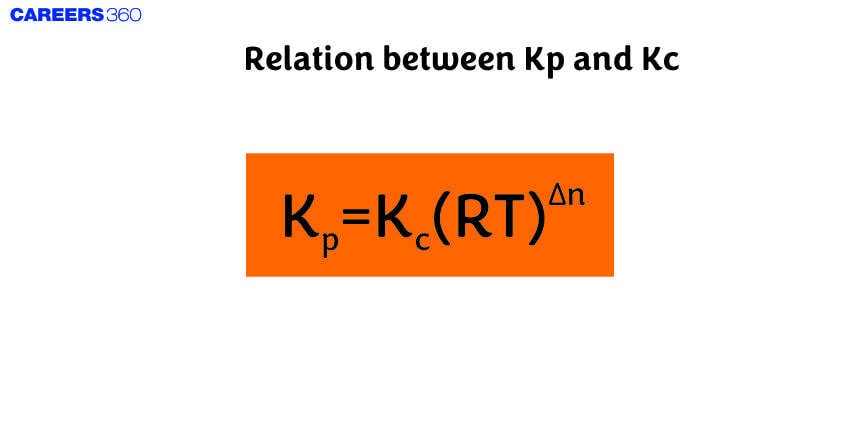
Kc is the equilibrium constant expressed in terms of the concentrations of reactants and products, while ( Kp ) is expressed in terms of the partial pressures of gases. The relationship between ( Kp ) and ( Kc ) is particularly important for understanding how changes in pressure and temperature affect chemical equilibria.
The specific relationship between ( Kp ) and ( Kc ) was derived later as the field of chemical thermodynamics evolved. The equilibrium constants ( Kp ) and ( Kc ) are extremely helpful in various ways. They provide insight into the extent to which a reaction will proceed to form products or remain as reactants. A large value of ( Kc ) or ( Kp ) indicates a reaction that favors the formation of products, while a small value suggests a reaction that favors the reactants.
Relation Between Kp And Kc
Let us suppose we react:
The equilibrium constant
For the reaction:
The equilibrium constant
Now, from the Ideal gas Equation
Putting the value of
Putting
It can be seen that:
- When
. When
When
Recommended topic video on(Relation between Kp and Kc)
Some Solved Examples
Example 1. An amount of solid NH4HS is placed in a flask already containing ammonia gas at a certain temperature and 0.50 atm pressure. Ammonium hydrogen sulphide decomposes to yield NH3 and H2S gases in the flask. When the decomposition reaction reaches equilibrium, the total pressure in the flask rises to 0.85 atm. The equilibrium constant forNH4HS decomposition at this temperature is
1)0.30
2)0.18
3) 0.17
4)0.11 (correct)
Total pressure = 0.5 + 2x = 0.84
x = 0.17 atm
Now,
Example 2. Two solids dissociate as follows
The total pressure when both the solids dissociate simultaneously is :
1)
2)
3)
4)
Solution
Relation between pressure and concentration -
or
or
- wherein
P is pressure in Pa. C is concentration in mol/liter. T is the temperature in kelvin
As we have learned in total pressure at equilibrium
Hence, the answer is the option (4).
Example 3. Consider the reaction
Solution
Now,
Due to
Total moles are 4,1 of
Partial Pressure of
Example 4. For the reaction,
1)
2)RT
3)
4) 1.0
Solution
Relation between
Now, for the given reaction,
Thus
Hence, the answer is the option (1).
Example 5. For the reaction,
1) -1
2) (correct)
3)
4) 1
Solution
We know that
Relation between Kp and Kc -
According to the data given in the question
For the given reaction
Hence, the value of
Hence, the answer is an option (2).
Summary
The relationship between ( Kc ) (the equilibrium constant for concentration) and ( Kp ) (the equilibrium constant for partial pressures) is crucial in understanding chemical equilibria, especially for reactions involving gases. This relation has various benefits such as predicting reaction behavior: Knowing the values of ( Kc ) and ( Kp ) helps to predict the direction and extent of a reaction under varying conditions.
Also Read
07 Feb'25 12:04 AM
07 Feb'25 12:00 AM
06 Feb'25 11:50 PM
06 Feb'25 11:48 PM
06 Feb'25 11:40 PM
09 Dec'24 11:40 AM
21 Oct'24 12:32 PM
21 Oct'24 11:57 AM
19 Oct'24 03:14 PM
19 Oct'24 03:08 PM
