Saturated Hydrocarbons - Definition, Examples, Types, Uses, FAQs
What is a Saturated Hydrocarbon?
The carbon-carbon bonds in a saturated hydrocarbon are all single bonds. Hydrocarbons are organic compounds whose only components are carbon and hydrogen. A saturated carbon compounds is a hydrocarbon in which all of its carbon atoms are bonded to at least four other atoms and are 'saturated,' implying that the organic compound contains no carbon-carbon multiple bonds.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is a Saturated Hydrocarbon?
- Saturated Hydrocarbon Types
- Alkanes
- Cycloalkanes
- Saturated and Unsaturated Hydrocarbons
- Saturated Hydrocarbons: What are their uses?
- Usages
Alkanes - acyclic hydrocarbons containing only carbon atoms with sp3 hybridization - are usually referred to as 'saturated hydrocarbons'. Alkanes have the general formula CnH2n+2. Below is an illustration of the structure of a propane molecule (C3H8).
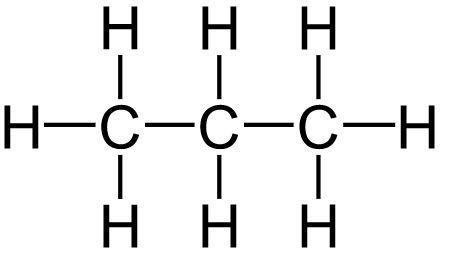
An observation is that a propane molecule has reached a point where it cannot accommodate any more hydrogen atoms in its parent chain. Here are a few more saturated hydrocarbon examples/saturated compounds examples;
Butane (C4H10)
Octane (C8H18)
Cyclohexane (C6H12)
Cyclopropane (C3H6)
Also read -
- NCERT Solutions for Class 11 Chemistry
- NCERT Solutions for Class 12 Chemistry
- NCERT Solutions for All Subjects
A cycloalkane is an alkane whose ring structure is monocyclic. A cycloalkane is considered a saturated hydrocarbon because it has only one carbon-carbon bond. The ring number in the molecule is represented by the letter 'r'.
Saturated Hydrocarbon Types
There are three major types of saturated hydrocarbons: linear, branched, and ring-shaped. Accordingly, they can be classified as follows:
Alkanes
Cycloalkanes
Polycyclic alkanes (alkanes whose structures include more than one ring) are categorized as cycloalkanes and, thus, are also saturated hydrocarbons.
Also read :
- NCERT notes Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic chemistry- some basic principles and techniques
- NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and techniques
Alkanes
The carbon chain in an alkane can be either linear or branched. In saturated hydrocarbons, the carbon atoms are all hybridized with sp3. In relation to the length of a carbon chain, an alkane's melting point and boiling point are determined. High melting and boiling points are associated with longer chains. Carbon chains have a high molecular weight because they have a long chain. Gases and liquids contain carbon atoms that are between 5 and 17 carbon atoms at standard temperatures. A room-temperature solid alkane with more than 18 carbon atoms is an alkane with more than 18 carbon atoms. It is known that chain isomerism occurs in alkanes containing at least three carbon atoms. Below is a diagram illustrating the possible chain isomers of butane(C4H10).
A polycyclic alkane has a finite number of chain isomers depending on how many carbon atoms are in it. The chemical makes only two chains isomers of butane, but there are 18 chains isomers of octane (C8H18).
Related Topics Link, |
Cycloalkanes
Those with cycloalkane properties are characterized by a ring-like arrangement of carbon atoms sp3. Nevertheless, the saturated hydrocarbon rings can branch into side chains. Cycloalkanes are somewhat similar to alkanes in terms of physical properties. It is generally accepted that cycloalkanes have higher melting and boiling points than alkanes of the same carbon atom count. Cycloalkanes exhibit ring strain due to their structure. All cycloalkanes have the same carbon-carbon bond angle, but cyclopropane has the highest ring strain due to its 60-degree angle.
Saturated and Unsaturated Hydrocarbons
Unlike saturated hydrocarbons, unsaturated hydrocarbons contain a double or triple carbon bond. Below is a table that compares saturated and unsaturated compounds hydrocarbons.
Difference between Saturated and Unsaturated Hydrocarbons
Saturated compounds hydrocarbon | Unsaturated compounds hydrocarbon |
The carbon atoms in these compounds are all sp3 hybridized. | The carbons are hybridized with sp2 or sp. |
The corresponding unsaturated hydrocarbons contain more hydrogen atoms. | Hydrocarbons contain fewer hydrogens than saturated hydrocarbons. |
There are alkanes and cycloalkanes as examples. | Examples of aromatic hydrocarbons include alkenes and alkynes. |
In terms of chemical reactivity, they are relatively inactive. | Compared to their saturated counterparts, they are more reactive. |
They generally burn with a blue flame. | They generally burn with a sooty flame. |
Saturated Hydrocarbons: What are their uses?
The alkane family is widely used as fuels, heating oils, and solvents. We list a few other applications for saturated hydrocarbons below.
In some automobiles, water heaters, and ovens, methane, the simplest alkane, is used as a fuel. Methane liquid is also suitable for rocket fuel in a highly refined form.
Ethane is used as a refrigerant in several cryogenic refrigeration systems. Ethylene is also produced from it.
A saturated hydrocarbon known as propane is the propellant used in some aerosol sprays. Fuel for hot air balloons is also made from this compound.
Besides preventing engine damage, octane is a very important component of gasoline.
In addition to motor fuels, diesel fuels, petroleum gases, and heavy oils, cycloalkanes are also used.
Cycloalkanes also play an important role in rubber and nylon manufacturing.
NCERT Chemistry Notes:
Usages
Hydrocarbons are predominantly used as combustible fuels. Natural gas is mainly composed of methane. Most gasoline, naphtha, jet fuels, and specialized industrial solvent mixtures contain C6 through C10 alkanes and alkenes, as well as isomeric cycloalkanes. Carbon units are introduced incrementally into simple non-ring structures to produce hydrocarbons with higher viscosities, lubricating indices, boiling points, solidification temperatures, and deeper colours. A crude oil refinery's lowest fraction is the heavy tarts that remain after methane is removed. Various materials derived from them are used as roofing compounds (bitumen), pavement compositions, and wood preservatives (the creosote series).
Also check-
Frequently Asked Questions (FAQs)
Hydrocarbons in which all carbon-carbon bonds are single bonds are saturated hydrocarbons. Carbon and hydrogen are the only components of a hydrocarbon.
In order to classify saturated hydrocarbons according to their structures, they are grouped into the following categories:
Alkanes
Cycloalkanes
The carbon chain in an alkane can be either linear or branched. In saturated hydrocarbons, the carbon atoms are all hybridized with sp3. Carbon chain length determines an alkane's melting and boiling points.
The alkane family of chemicals is extensively used for fuel, heating oil, and solvents. We list a few other applications for saturated hydrocarbons below.
In addition to gasoline, methane is used for heating water, powering water heaters, and powering ovens. Methane liquid is also suitable for rocket fuel in highly refined form.
Ethane is used as a refrigerant in a number of cryogenic refrigeration systems. Ethylene is also produced from it.
Those with cycloalkane properties are characterized by a ring-like arrangement of carbon atoms sp3. Nevertheless, side chains can branch off from the ring in these saturated hydrocarbons.
Also Read
12 Dec'24 04:49 PM
04 Nov'24 05:31 PM
21 Oct'24 05:53 PM
09 Oct'24 06:12 PM
09 Oct'24 04:00 PM
30 Sep'24 10:57 AM
30 Sep'24 08:52 AM