Sigma and Pi Bond - Formation, Examples, Overlapping with FAQs
Have you ever wondered why certain molecules form stable bonds while others don’t? And What makes a single bond different from a double or triple bond? The answer to these questions is Sigma and Pi Bond formation and their overlapping. There are various types of bonding among atoms, but sigma and pi bonds are fundamental bonds that are the main force behind the structure and stability of the molecule. When there is direct or head-on overlapping of atomic orbitals, then a sigma bond is formed, which represents the strongest type of covalent bond and forms the basis of molecular structures. While sideways overlapping of atomic orbitals leads to the formation of pi-bonds. Pi-bonds are always accompanied by sigma bonds in double and triple bonds.
This Story also Contains
- Formation Of Sigma And Pi Bond
- The Sigma (σ) Bond
- The Pi (π) Bond
- What Are Sigma And Pi Bonds?
- Types Of Overlapping
- Organometallic Compounds
- Sigma-Bonded Organometallic Compounds
- Pi-Bonded Organometallic Compounds
- Sigma Bond vs Pi Bond:
- Some Solved Examples
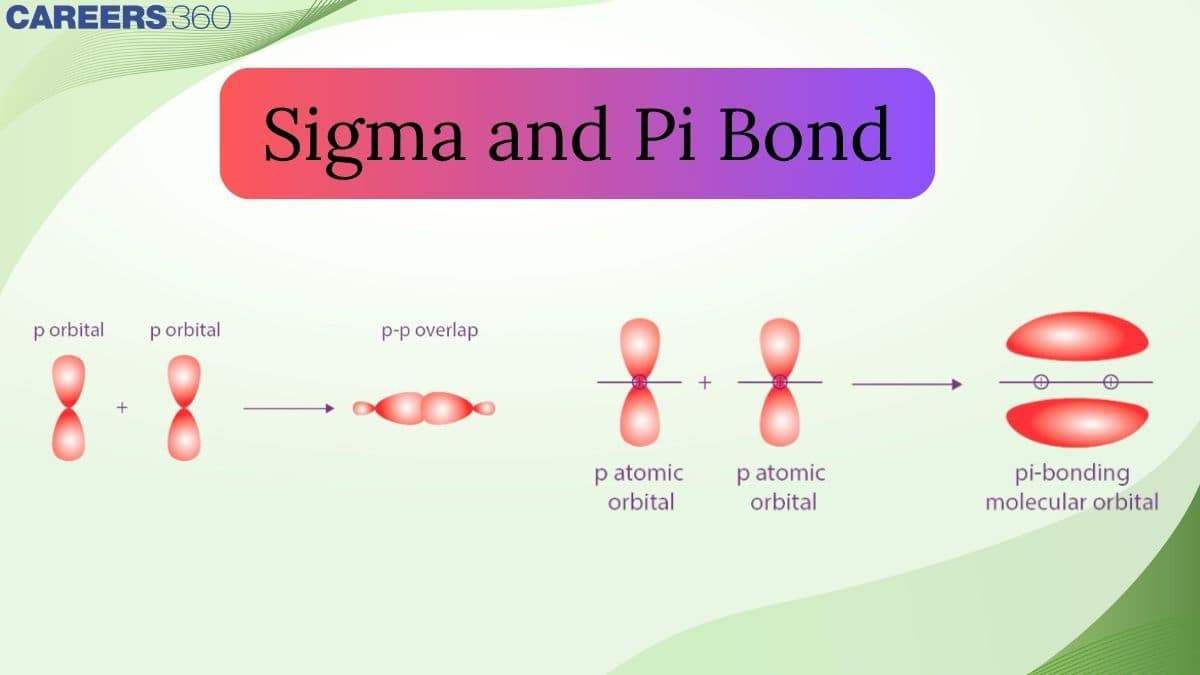
For example, a doubly bonded oxygen molecule has one sigma bond and one pi bond, while a triple-bonded N2 molecule has 1 sigma bond and two pi bonds. Everything around us, like oxygen in air, atoms of carbon in polythene, carbon in coal or diamond, is bonded in one way or another. The strengths and weaknesses of elements are based on the type of bonding between the atoms of that element.
Formation Of Sigma And Pi Bond
The hybridization helps in predicting such complex models of squeezing electrons into the same space of a sphere by concluding all the Sigma Bonds. The structure of ethane consists of C-C single bonds and C-H single bonds, while the structure of ethene has a double bond between C-C and a single bond between C-H, and so the geometry of such a covalent compound is planar. In the above figure, the hybridization found is sp2 where whereas in the case of ethane hybridization was sp3.
Thus, we can see that in ethene generation of 2pz unhybridized orbital with each orbital containing one electron, which is capable of forming a covalent bond. The bonding in ethene can be summarized as follows: The three sp2 hybrids overlap with other orbitals of the carbon atom that are identical. The remaining two hybrid orbitals form the bond with the hydrogen atom by overlapping with the 1s orbital. Hence, the 2pz on the carbon atom forms the other bond at a sideways of one another.
.png)
The Sigma (σ) Bond
The single bond formed is termed a Sigma Bond. A sigma bond can be represented as "σ". A sigma (σ) bond is the strongest type of covalent bond formed by the head-on (axial) overlap of atomic orbitals. The bond formed by the cation of end-to-end overlap of sigma bonds, where the concentrated density of electrons was found in the nuclei of atoms and the bonding atoms. Sigma bonds are formed end-to-end, which means the overlapping is done coaxially. Such type of overlapping is called axial overlapping.
Example Of Sigma Bond
A sigma bond can be seen in the formation of a hydrogen molecule. This can be seen in the picture depicted below, where hydrogen is bonded singly as a sigma bond.
The Pi (π) Bond
The pi bond is the second bond that is formed in between the C-C atoms and is elongated both above and below the plane of the molecule. According to Lewis dot structure, a double bond, which is formed between molecules, can be represented by a single dash. Pi bond can be represented as π. In a pi bond, the electron density is concentrated on both above and below the plane of the nuclei of the bonding atoms. Therefore, it is noted that the two bonds are formed sigma bond and a pi bond, in the covalent molecule of ethene. In general, when a single bond is present between the two atoms, it is a sigma bond, whereas double bonds consist of one sigma bond and one pi bond. In the case of triple bonds, one sigma and two pi bonds are present.
Examples Of Pi bonds
Ethyne bonding is the ones such example of pi bonding, which is singly bonded with one hydrogen atom and triple bonds are present mong carbon atoms. The bonds are formed by electron transfer of specific wavelength of 2s electron to 2pz. Here, one 2s orbital will combine with one 2p orbital to form a sigma bond and sp hybridization, whereas the remaining 2py and 2pz form the pi bond.
What Are Sigma And Pi Bonds?
The names are derived from the Greek letters. The sigma and pi bonds are both used to predict the molecule's behaviour as according to molecular orbital theory. In a sigma bond, the orbitals are as similar as according to “s” where whereas in a pi bond, the orbital is similar to the p orbitals. It is found that sigma bonds are stronger in comparison with pi bonds.
Types Of Overlapping
Direct overlapping can be seen in sigma bonds. The electrons that participate in bond formation are termed sigma electrons. Singly bonded electrons are formed according to the combination of atomic orbitals as follows:
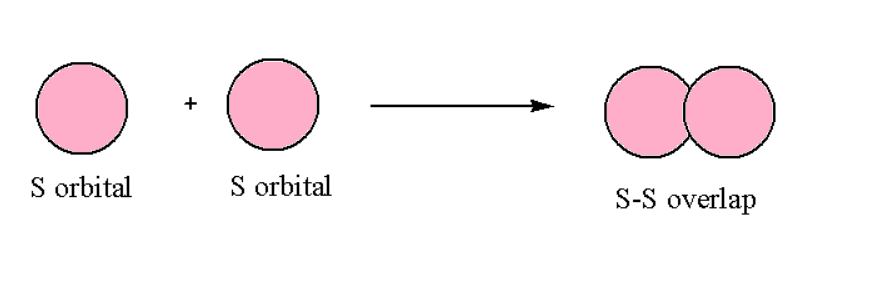
s-s Overlapping
In such a type of overlapping, one‘s orbital will participate along the internuclear axis, which means head-on overlapping. This can be represented as follows:
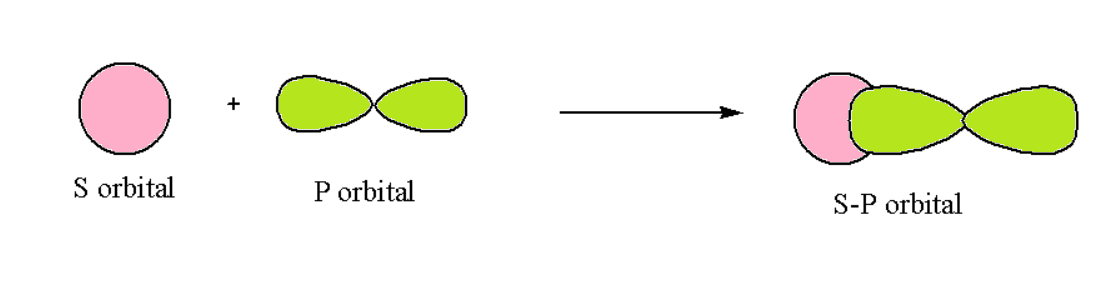
s-p Overlapping
In such a type of overlapping occurs along the internuclear axis when one half-filled p orbital overlaps with one half-filled orbital, and forms the covalent bond. Such a condition can be represented as follows:
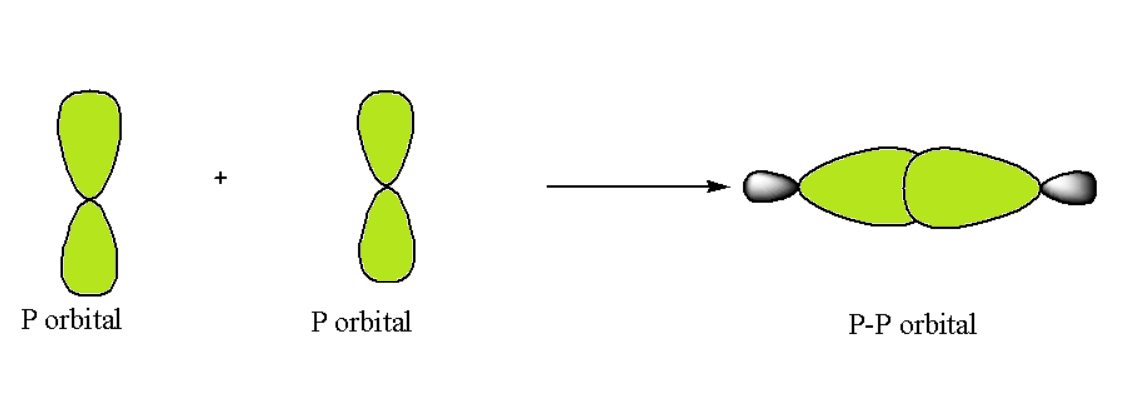
p-p Overlapping
In such a type of overlapping occurs along the internuclear axis when one half-filled orbital undergoes head-on overlapping. Such a condition can be represented as follows:
Organometallic Compounds
Sigma-bonded organometallic compounds are defined as those compounds that are formed with the help of metal ‘M’ and carbon of the ligands containing a covalent sigma bond, and are termed as sigma-bonded organometallic compounds. Here, the metal can be bonded to any alkyl family, aryl family, or pi-bonded ligands. These organometallic compounds are basically divided according to the metal-carbon bond as follows:
-
Sigma-bonded organometallic compounds
-
Pi-bonded organometallic compounds
Sigma-Bonded Organometallic Compounds
The sigma-bonded organometallic compounds are those compounds that contain a metal-carbon covalent sigma bond, so they are termed as sigma-bonded organometallic compounds. Examples of such sigma-bonded organometallic compounds are Grignard reagents, which have a general structure as R-Mg-X, where R is an organic group and X is a halogen compound. In this example covalent sigma bond is present in metal, that is, magnesium, and R is any aryl or alkyl.
Pi-Bonded Organometallic Compounds
Taking an example of ferrocene compound, in which the two Fe- (C2H5) bonds. The compound Fe is bonded in a pi bond system; thus, ferrocene is in pi-bonded organometallic compound.
Sigma Bond vs Pi Bond:
|
Parameters |
Sigma Bond |
Pi Bond |
|
Bond Formation |
The sigma bonds are formed as axial overlap of atomic orbitals. |
The pi bonds are formed as lateral overlapping of atomic orbitals. |
|
Overlapping orbitals |
Sigma bonds overlap of bonds: one hybrid orbital with two hybrid orbitals or one pure orbital with two hybrid orbitals. |
Pi bonds overlap of bonds: alternating orbitals. |
|
Existence |
Independent |
Always with sigma bonds |
|
Bond strength |
Stronger than pi bonds |
Less stronger than sigma bonds |
|
Number of bonds |
Single sigma bond between two atoms |
Two pi bonds between two atoms |
|
Number of bonds in a double bond |
One sigma bond is present in a double bond. |
Only one pi bond is present in a double bond |
|
Number of bonds in a triply bonded atom |
One sigma bond in a triply bonded atom |
Two pi bonds are there in triply bonded atoms. |
|
Shape of the molecule |
Shape can be determined by the sigma bond |
No determination of shape is possible through pi bonds. |
|
Order of formation of bonds |
First, sigma bonds are formed when atoms come closer together |
First, the sigma bonds are formed, then the pi bonds |
Read more
- NCERT notes Class 11 Chemistry Chapter 12 Organic Chemistry- Some Basic Principles and Techniques
- NCERT solutions for Class 11 Chemistry Chapter 12 Organic Chemistry- Some Basic Principles and
techniques - NCERT Exemplar Class 11 Chemistry Solutions Chapter 12 Organic chemistry- some basic principles and
techniques
Some Solved Examples
Question.1 XeO4 molecular is tetrahedral having:
1) Two $p \pi-d \pi$ bonds
2) One $p \pi-d \pi$ bond
3) Four $p \pi-d \pi$ bonds
4) Three $p \pi-d \pi$ bonds
Solution:
One s and three p orbital undergo sp3 hybridization. Four sp3 hybrid orbitals form four σ bonds with oxygen atoms. They are σ sp3 – p. Four pπ – dπ bonds are also formed with oxygen atoms by the unpaired electrons.
Hence, the answer is option (3).
Question.2 Among $\mathrm{P}_2 \mathrm{O}_5$ and $\mathrm{P}_4 \mathrm{O}_{10}$ the compound having more number of $\sigma$ and $\pi$ bond respectively are :
1) $\mathrm{P}_2 \mathrm{O}_5, \mathrm{P}_4 \mathrm{O}_{10}$
2) $\mathrm{P}_4 \mathrm{O}_{10}, \mathrm{P}_2 \mathrm{O}_5$
3) $\mathrm{P}_4 \mathrm{O}_{10}, \mathrm{P}_4 \mathrm{O}_{10}$
4) None of these
Solution:
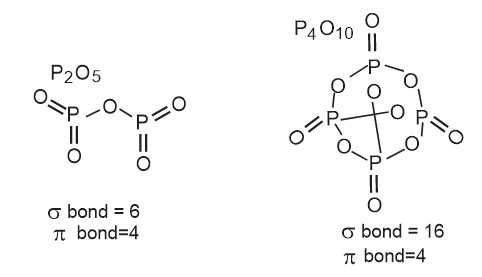
Hence, the answer is option (4).
Question.3 Which of the following molecules has two sigma $(\sigma)$ and two $\mathrm{pi}(\pi)$ bonds ?
1) $\mathrm{C}_2 \mathrm{H}_4$
2) $N_2 F_2$
3) $\mathrm{C}_2 \mathrm{H}_2 \mathrm{Cl}_2$
4) HCN
Solution:
$\mathrm{C}_2 \mathrm{H}_4$ has $5 \sigma$ and one $\pi$ bond
$\mathrm{N}_2 \mathrm{~F}_2$ has $3 \sigma$ and one $\pi$ bond
$\mathrm{C}_2 \mathrm{H}_2 \mathrm{Cl}_2$ has $5 \sigma$ and one $\pi$ bond
HCN has $2 \sigma$ and two $\pi$ bond
Hence, the answer is option (4).
Practice More Questions from the Link Given Below in the Table
Also read -
Frequently Asked Questions (FAQs)
Pi-bonds are formed by the sideways overlapping of atomic orbitals. Pi-bonds are always accompanied by sigma bonds in double and triple bonds.
No, a molecule cannot have only a pi bond; they are always accompanied by sigma bonds. For example, in a doubly bonded molecule, one bond is a sigma bond and the other bond is a pi bond, while in a triply bonded molecule, one bond is a sigma bond, while the other 2 bonds are pi bonds.
Sigma and pi bonds together influence the geometry of molecules. Sigma bonds can rotate freely around the bond axis, allowing for a range of conformations. However, pi bonds restrict this rotation due to their electron clouds being located above and below the bond axis, creating a planar structure. This essential difference in behavior helps define the overall shape of the molecule, impacting its chemical properties and reactivity.
Sigma bonds are strong bonds, and they are the first bonds formed between atoms. They form when there is direct or head-on overlapping of atomic orbitals.
Pi bonds are weak bonds. Pi-bonds are formed by the sideways overlapping of atomic orbitals.
