Surface Chemistry
Introduction
Think of a non-stick pan versus a regular one—the work of surface chemistry is directly proportional to how easily the food slides out from a non-stick pan. Be it water-repellent coatings on our phones or adhesives in band-aids, surface chemistry impresses on such diverse aspects of our lives. Such everyday applications hammer home very strongly how important it is to understand molecular interactions going on at surfaces and interfaces.
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- Introduction
- Types and Aspects of Surface Chemistry
- Surface Modification
- Academic and Industrial Importance
It concerns that part of chemistry is connected with the physical and chemical phenomena happening at surfaces and interfaces, studying phenomena like adsorption, catalysis, and surface tension. The following paper gives an overview of the basics of the area of Surface Chemistry and its various subfields, along with its real significance in applications. The interactions between atoms and molecules on a surface are, obviously, complex in nature; therefore, knowledge about these interactions makes headway into the fields of materials science, nanotechnology, and even biotechnology.
Definition and General Description
Surface chemistry is the branch of chemistry concerned with the study of the processes occurring at surfaces and interfaces of materials. Mostly, it deals with the interaction of molecules with solid surfaces and the changes going on during such interactions. Key amongst these include:
- Adsorption: Molecules build up on the surface.
- Catalysis: Acceleration of a chemical reaction because of the presence of the catalyst at the surface.
- Surface Tension: Work done to isothermally increase the surface area of a liquid.
Role of Interfaces
For any two homogeneous phases, there will always exist boundaries between the two phases. It is the nature of these interfaces that defines many important questions about materials and reactions. For example, for most catalysts, it is observed that the effectiveness in most cases to a great extent is dependent upon the surface area presented to the reaction.
As the name suggests, Surface chemistry is the study of chemical reactions at surfaces and interfaces. Surface chemistry tells us how molecules and atoms interact with surfaces and with each other while on surfaces. There are various phenomena taking place on the surface, but we will limit ourselves to learn about basic phenomena like adsorption, Heterogeneous Catalysis, Corrosion, and Crystallisation.
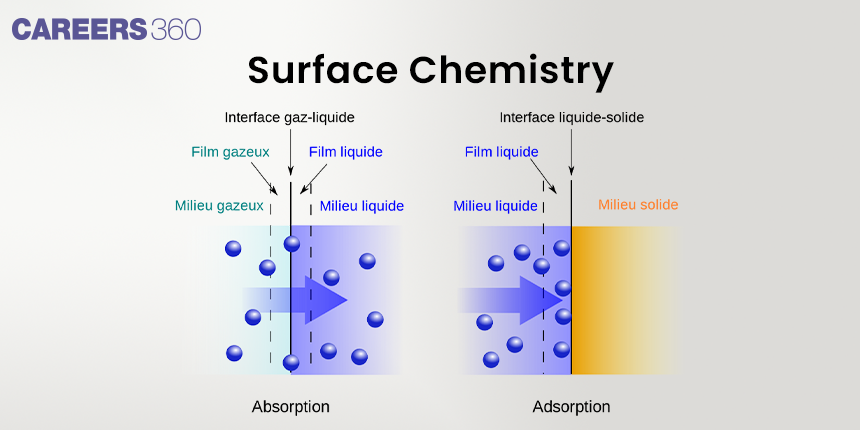
The two bulk phases can be pure compounds or solutions. The interface is represented by a Hyphen or a slash between the two bulk phases involved. e.g. - Solid-Liquid. No interface exists between gases as they are completely miscible with each other.
Types and Aspects of Surface Chemistry
Adsorption
Adsorption is a process whereby the gas or liquid molecules attach themselves to the solid or liquid surface. Basically, there exist two types of adsorption divided into:
- Physisorption: It is a kind of weak Van der Waals force.
- Chemisorption: It involves stronger chemical bonds between adsorbate and adsorbent.
Catalysis
Specifically, catalysts increase the rate of a chemical reaction without themselves undergoing any change. Surface Catalysts: These are a type of catalyst used to reduce the effect of byproducts of combustion, which is harmful, and gives a less harmful gas. This is often fitted in the car catalytic converters.
Surface Modification
Surface modification refers to the process of attempting to change the surface properties of a material for desired characteristics. These techniques include coating, etching, functionalization, etc., to enhance properties such as hydrophobicity, conductivity, and biocompatibility.
Relevance and Applications
Practical Applications
Applications of surface chemistry are seen in the following areas:
- Medical Devices: Surface modification makes them more biocompatible.
- Environmental Protection: Catalysts help to reduce pollutants.
- Electronics: Performance improvement in semiconductors through surface engineering.
Academic and Industrial Importance
To this respect, function-connected academic levels of research in the field of surface chemistry lead to new materials and new technologies. This understanding also enhances the quality and performance of any product in the industry. Hence, innovation in fields like nanotechnology and materials science would not have been possible without an understanding of the interactions at surfaces.
Recommended topic video on ( Surface Chemistry)
Some Solved Examples
Example 1
Question:
Which process does not occur at the interface of the phases?
1. Crystallisation
2. Heterogeneous catalysis
3. Corrosion
4. Homogeneous Catalysis (Correct Answer)
Solution:
In surface chemistry, processes that occur at the interface of phases involve interactions between different states of matter, such as solid, liquid, and gas. Homogeneous catalysis, however, occurs within a single phase, where the catalyst and reactants are in the same phase, usually a solution. Therefore, homogeneous catalysis does not involve an interface between different phases, making option 4 the correct answer.
Example 2
Question:
In which of the following, interfaces can not be obtained?
1. Liquid-Liquid
2. Liquid-Gas
3. Solid-Liquid
4. Gas-Gas (Correct Answer)
Solution:
An interface is a boundary between two different phases of matter. In the case of a gas-gas mixture, gases are completely miscible with each other and do not form a distinct boundary or interface. Therefore, option 4 is correct because there is no interface between two gases.
Summary
Surface chemistry is the study of various chemical phenomena taking place at surfaces and interfaces. If anything, surface chemistry has driven many applications. Better knowledge of these molecular interactions may lead to major improvements in such diverse areas as the non-stick properties of frying pans and the biocompatibility of medical tools. Core concepts include adsorption, catalysis, and surface tension, all bearing on the determination of the behavior of materials and reactions at interfaces. Applications of surface chemistry go both into academic research and practical applications, hence become very important to power technological innovation.
Also Read
19 Feb'25 04:59 PM
04 Nov'24 10:45 AM
07 Oct'24 12:46 PM
07 Oct'24 12:44 PM
04 Oct'24 06:04 PM
30 Sep'24 02:35 PM
30 Sep'24 02:28 PM
30 Sep'24 11:36 AM