Third law of Thermodynamics
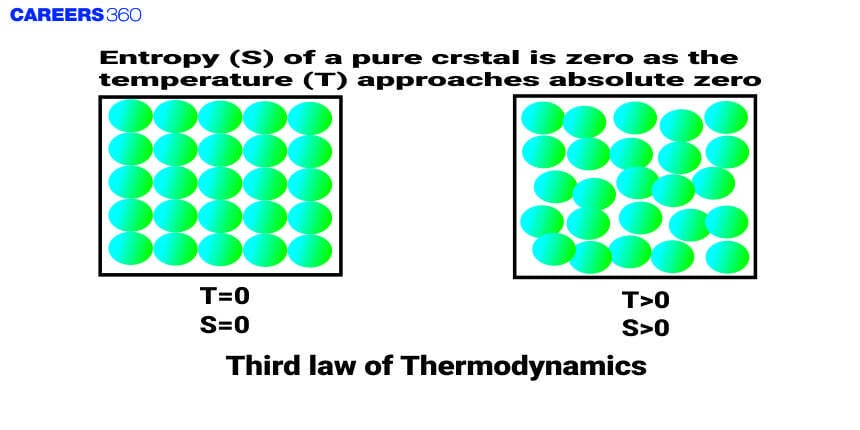
The Third law of Thermodynamics is also known as the Nernst Theorem. It was discovered by the German chemist Walter Nernst in 1906. This law states that as the temperature of the system approaches absolute zero, the entropy of a perfectly crystalline substance approaches zero.
The third law of thermodynamics states that the entropy of a perfectly crystalline solid at absolute zero temperature(zero kelvin) is equal to zero. Entropy is called the randomness of the particles or disorder in the closed system and it is denoted by the S.
Mathematical Explanation Of Third Law
The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero. This is called the third law of thermodynamics.
- At any pressure, the entropy of every crystalline solid in thermodynamic equilibrium at absolute zero is zero.
- It is impossible to reduce the temperature of any system to absolute zero by any process.
- As the absolute temperature approaches zero, the increment in entropy for the isothermal process in crystalline solids approaches zero,
- Mathematical Explanation Of Third Law
- Some Solved Examples
- Summary
i.e. S=0 at T=0
Or limT→0,S→0
If the molar heat capacities of a substance (Cp) are measured at different temperatures a graph between Cp/T vs T is drawn and it shows this type of behavior.

Now let SMo be the entropy of the substance at zero Kelvin and SM is its molar entropy at Kelvin then
ΔS=SM−SMo∴ΔS=SM(∵SMo=0, according to 3rd Law of thermodynamics) ΔS=qTΔS=∫0TCp⋅dTT,∵q=1×Cp⋅dT∴ΔS=SM=∫0TCp⋅dTT
The area under the curve or graph of Cp/T vs T determined from zero Kelvin to any desired temperature would be molar entropy change going from zero to desired T.
Recommended topic video on (Third law of Thermodynamics)
Some Solved Examples
Example 1. In defining the temperature scale, the standard reference point is taken as:
1)0 Kelvin
2)boiling point of water
3) (correct)Triple point of water
4)None of the above
Solution
In defining the temperature scale, the standard reference point is taken as the triple point of water.
Hence, the answer is the option (3)
Example 2. Which law states, “It is impossible to reduce any system to the absolute zero of temperature in a finite number of operations”.
1)1st law of Thermodynamics
2)2nd law of Thermodynamics
3) (correct)3rd law of Thermodynamics
4)None of the above
Solution
3rd law of thermodynamics states: The entropy change associated with any condensed system undergoing a reversible isothermal process approaches zero as the temperature at which it is performed approaches 0 K. It also says: It is impossible for any process, no matter how idealized, to reduce the entropy of a system to its absolute zero value in a finite number of operations.
Hence, the answer is the option (3)
Example 3. At absolute zero temperature, the entropy of a pure crystal is:
1) (correct)Zero
2)Positive
3)Negative
4)Any of the above
Solution
As we have learnt
3rd Law Of Thermodynamics -
The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero. This is called the third law of thermodynamics.
- At any pressure, the entropy of every crystalline solid in thermodynamic equilibrium at absolute zero is zero.
- It is impossible to reduce the temperature of any system to absolute zero by any process.
- As the absolute temperature approaches zero, the increment in entropy for the isothermal process in crystalline solids approaches zero,
i.e. S=0 at T=0
limT→0, S→0
According to the third law of thermodynamics, the entropy of pure crystal is zero at absolute zero temperature.
Therefore, Option(1) is correct.
Example 4. Which of the following laws was formulated by Nernst?
1) First law of thermodynamics
2) Second law of thermodynamics
3) (Correct)Third law of thermodynamics
4) None of the above
Solution
As we have learnt
3rd Law Of Thermodynamics -
The entropy of any pure crystalline substance approaches zero as the temperature approaches absolute zero. This is called the third law of thermodynamics. and it is discovered by Walter Nernst.
Therefore option (3) is correct.
Summary
The law helps in calculating the absolute entropy of a system and understanding the behaviour of various thermodynamic processes. It is very important to understand the material study at very low temperatures including the superconductor and many more.
Also Read
02 Jul'25 04:56 PM
02 Jul'25 04:56 PM
02 Jul'25 04:55 PM
02 Jul'25 04:50 PM
02 Jul'25 04:45 PM
02 Jul'25 04:43 PM
02 Jul'25 04:41 PM
02 Jul'25 04:33 PM
02 Jul'25 04:32 PM