Van Der Waals Forces: Definition, Formula, Examples, Questions
The forces that hold atoms together within a molecule are strong covalent or ionic bonds, the Van der Waals forces are relatively weak, consisting of transitory dipoles induced by the movement of electrons. These forces hold the key to physical material properties, the structure of biological macromolecules, and interactions between different substances.
This Story also Contains
- Understanding Van der Waals Forces
- Van der Waal Forces
- Recommended topic video on ( Vander Waals Forces)
- Some Solved Examples
- Summary

Understanding Van der Waals Forces
Van der Waals forces are intermolecular forces that are weak and result from the interactions between uncharged atoms or molecules. They depend on the distance of the molecules from one another and are generally divided into three types: London dispersion forces, dipole-dipole interaction, and dipole-induced dipole interaction.
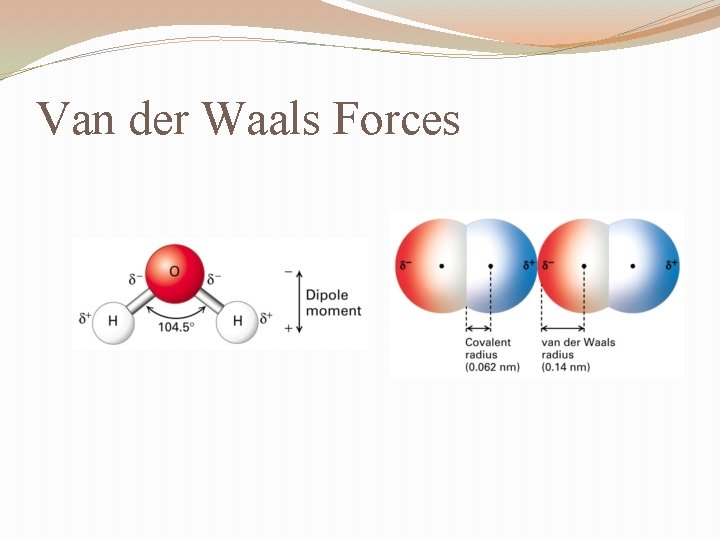
Van der Waal Forces
Van der Waal's force of attraction is the force of attraction between the molecules. This force is weaker compared to bonds like covalent and ionic bonds.
Van der Waal forces can be divided into various categories as follows:
Ion-dipole interaction: This type of interaction exists between an ion and a polar molecule like HF, HCl, H2O, etc. The ion can be like Na+. This type of interaction is responsible for the dissolution of ions in solution.
Dipole-Dipole interaction: This type of interaction exists between two or more polar molecules. These dipoles can be H-Cl and H-Cl, NH3 and NF3, etc. Hydrogen bonding is a special type of dipole-dipole interaction.
Ion-induced dipole interaction: This type of interaction exists between the ion and non-polar molecule. The charge on the ion distorts the electron cloud of the non-polar molecule and thus induces a dipole in the non-polar molecule. Then the ion and the induced dipole attract each other.
Dipole-induced dipole interaction: This kind of interaction exists between a polar and a non-polar molecule. For example, CCl4 in H2O. One of the dipole molecules distorts the electron cloud in the non-polar molecule and thus creates the dipole in the non-polar as well.
Instantaneous dipole-dipole interaction: This type of interaction exists between two non-polar molecules. This force is also known as the London forces. At any instant, electrons in one non-polar molecule come closer to each other and then this molecule becomes a dipole for instance. This instantaneous dipole distorts the electron cloud in another non-polar molecule and thus both behave like polar molecules. For example CCl4 and CCl4.
The strength of these forces follows the given order:
Ion-Dipole > Dipole-Dipole > Ion-Induced Dipole > Dipole-Induced Dipole > London Forces
Recommended topic video on ( Vander Waals Forces)
Some Solved Examples
Example 1
Question: The strength of the hydrogen bond is intermediate between which of the following?
1) Van der Waals and covalent
2) Ionic and covalent
3) Ionic and metallic
4) Metallic and covalent
Solution: The strength of hydrogen bonds is indeed intermediate between Van der Waals forces and strong covalent bonds. Therefore, the correct answer is option (1).
Example 2
Question: HF has the highest boiling point among hydrogen halides because it has:
1) Strongest Van der Waals interaction
2) Lowest ionic character
3) Strongest hydrogen bonding
4) Lowest dissociation enthalpy
Solution: HF exhibits the strongest hydrogen bonding among hydrogen halides, which significantly raises its boiling point compared to others. Thus, the correct answer is option (3).
Example 3
Question: The interaction energy of London forces between two particles is proportional to rx , where r is the distance between the particles. What is the value of x?
1) 6
2) -6
3) 3
4) -3
Solution: The interaction energy of London forces is proportional to 1r6, indicating that x = -6. Therefore, the correct answer is option (2).
Example 4
Question: Which of the following interactions is the strongest?
1) London dispersion forces
2) Dipole-dipole interactions
3) Hydrogen bonds
4) Ion-dipole interactions
Solution: Ion-dipole interactions are the strongest among the listed types of interactions. Hence, the correct answer is option (4).
Example 5
Question: In which of the following scenarios would you expect dipole-induced dipole interactions to occur?
1) Between two polar molecules
2) Between a polar molecule and a nonpolar molecule
3) Between two nonpolar molecules
4) Between two ionic compounds
Solution: Dipole-induced dipole interactions occur between a polar molecule and a nonpolar molecule. Therefore, the correct answer is option (2).
Summary
The most frequently overlooked bonds in chemistry are the van der Waals forces. They form the basis for an understanding of molecular interactions and the behavior of matter. These are weak intermolecular forces due to the interactions between uncharged molecules: London dispersion forces, dipole-dipole interactions, and dipole-induced dipole forces.