VSEPR Theory: Definition, Table, Chart, Formula, Examples, Questions
According to this theory, electron pairs around a central atom will arrange themselves as far apart as possible to minimize repulsion. VSEPR considers both bonding electron pairs—pairs of electrons that are involved in the formation of bonds—and lone pairs of electrons that do not participate in bonding when predicting the geometry of a molecule. The common molecular geometries that result from VSEPR include linear, trigonal planar, tetrahedral, trigonal bipyramidal, and octahedral.
This Story also Contains
- VSEPR Theory
- Practice More Questions From the Link Given Below:
- Summary
In this article, we will cover the concept of VSEPR Theory. This concept falls under the broader category of Chemical Bonding, which is a crucial chapter in Class 11 chemistry. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE, and more.
Also Read -
VSEPR Theory
Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom of a molecule from the estimation of the number of bonds and lone pairs of electrons in its Lewis structure.
The main postulates of VSEPR theory are:
-
The actual shape of a molecule depends upon the number of electron pairs (bonded or non–bonded) around the central atom.
-
The electron pairs tend to repel each other due to their negative charge.
-
Electron pairs arrange themselves in such a way that there exists a minimum repulsion between them.
-
The valence shell is considered as a sphere with the electron pairs placed at a distance.
-
A multiple bond is treated as if it is a single electron pair & the electron pairs that constitute the bond as a single pair.
-
The repulsive interaction of electron pairs decreases in the order as mentioned below:
Lone pair (lp) – Lone pair (lp) > Lone pair (lp) – Bond pair (bp) > Bond pair (bp) – Bond pair (bp).
-
Double bonds cause more repulsion than single bonds, and triple bonds cause more repulsion than double bonds. This repulsion decreases sharply with increasing bond angle between the electron pairs.
Let us understand VSEPR theory using a gaseous BeF2 molecule. In the Lewis structure of BeF2 as shown in the figure, there are only two electron pairs around the central beryllium atom. With two bonds and no lone pairs of electrons on the central atom, the bonds are as far apart as possible, and the electrostatic repulsion between these regions of high electron density is reduced to a minimum when they are on opposite sides of the central atom, thus the bond angle is 180°.

The BeF2 molecule adopts a linear structure in which the two bonds are at maximum distance from each other and maintain an angle of 180°.
Nyholm and Gillespie in 1957, refined the VSEPR model by explaining the important difference between the lone pairs and bonding pairs of electrons. They postulated that bond pair of electrons is shared by the two atoms whereas lone pair is under the influence of only central atom. As a result, in a molecule, the electron cloudcontaining lone pair is more spread out and occupy more space as compared to the electron cloud containing bond pair. This causes relatively greater repulsive interactions between the lone pairs in comparison to lone pair-bond pair repulsive interactions.
These repulsive effects result in deviations from idealised shapes and alterations in bond angles in molecules.
For the prediction of geometrical shapes of molecules with the help of VSEPR theory, it is convenient to divide molecules into two categories as:
(i) Molecules in which the central atom has no lone pair.
(ii) Molecules in which the central atom has one or more lone pairs.
First category molecules possess regular geometry and the second category molecules have irregular or distorted geometry.
The table given below shows the arrangement of electron pairs about a central atom A (without any lone pairs) and geometries of some molecules/ions of the type AB.
.jpg)
As per the table shown as above in compounds of AB2, AB3, AB4, AB5 and AB6, the arrangement of electron pairs and the B atoms around the central atom A are: linear, trigonal planar, tetrahedral, trigonal-bipyramidal and octahedral, respectively. Such arrangement can be seen in the molecules like BF3(AB3), CH4(AB4) and PCl5(AB5) as shown below by their ball and stick model.
.jpg)
The table given below shows the shapes of some simple molecules and ions in which the central atom has one or more lone pairs.
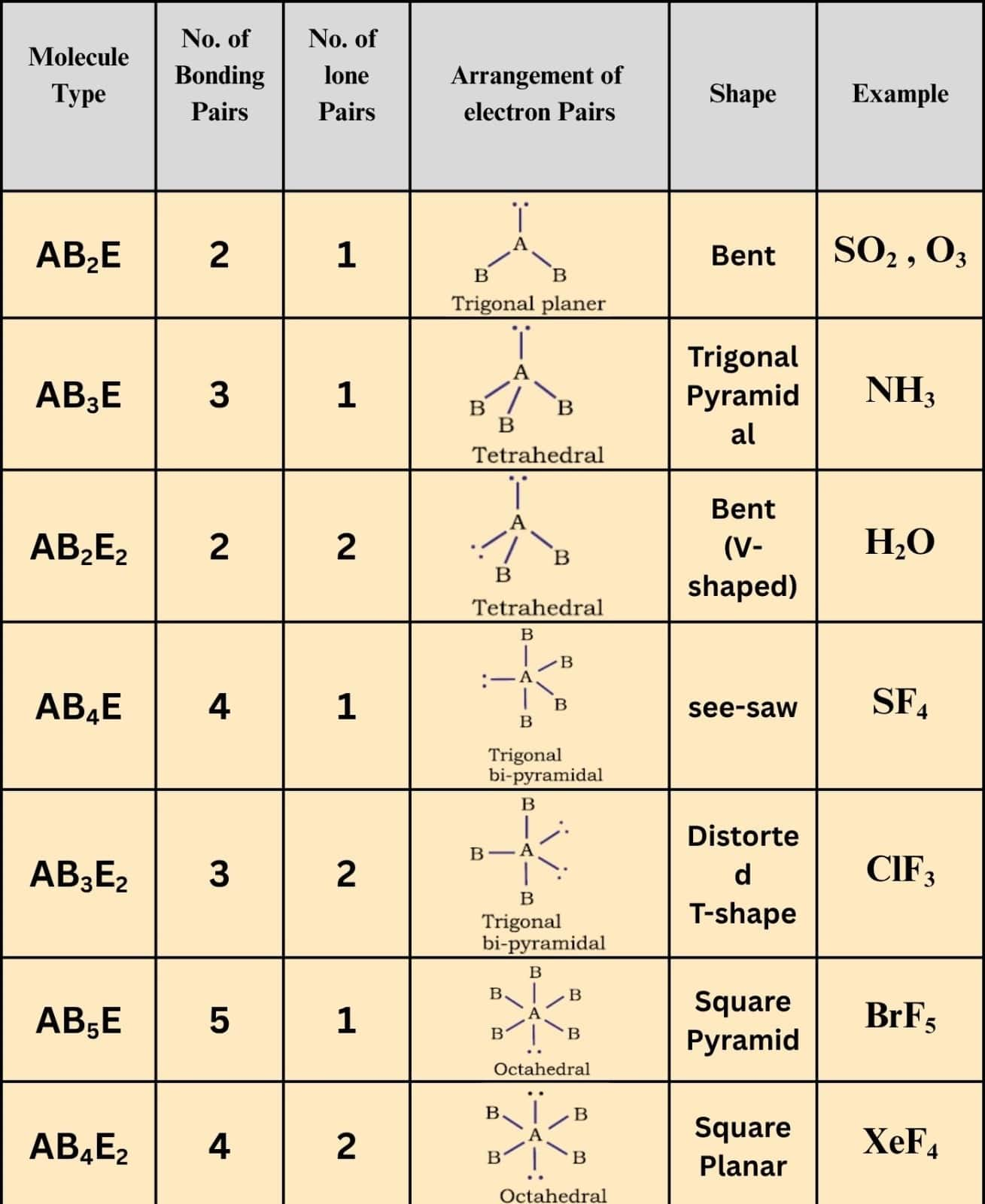
As given in the table below, two regions of electron density around a central atom in a molecule form a linear geometry, three regions form a trigonal planar geometry, four regions form a tetrahedral geometry, five regions form a trigonal bipyramidal geometry, and six regions form an octahedral geometry.

Related Topics-
Some Solved Examples
Example 1: The molecule having smallest bond angle is :
1)NCl3
2)AsCl3
3) SbCl3
4)PCl3
Solution
As we discussed in the concept
VSEPR Theory -
1. The shape of the molecule is determined by repulsions between all of the electron pairs present in the valence shell.
2. Order of repulsion
lone pair - Lone pair > Lone pair - Bond pair > Bond pair - bond pair
3 Repulsion among the bond pair is directly proportional to the bond order and electronegativity difference between the central atom and the other atom.
As we move down the group the size of the atom increases and as the size of the central atom increases lone pair-bond pair repulsion also increases. Thus bond angle decreases.
Increasing order of atomic radius:
N<P<As<Sb
Decreasing order of bond angle :
NCl3>PCl3>AsCl3>SbCl3
Hence, the answer is the option (3).
Example 2: The correct order of bond angles (smallest first ) in H2 S,NH3,BF3, and SiH4 is
1) H2 S<SiH4<NH3<BFF3
2) NH3<H2 S<SiH4<BF3
3) H2 S<NH3<SiH4<BF3
4) H2 S<NH3<BF3<SiH4
Solution
The correct order of bond angle ( smallest first )is
H2S<NH3<SiH4<BF392.6∘<107∘<109∘28′<120∘

Bond angle order = H2S < NH3 < SiH4 < BF3
Example 3: The type of hybridization and number of lone pair (s) of electrons of Xe in XeOF4 respectively, are :
1) sp3d2 and 1
2) sp3d and 2
3) sp3d2 and 2
4) sp3d and 1
Solution
As we have learnt in Hybridisation:

Hybridization is sp3d2
It contains 5 sigma bonds and 1 pi bond.
It has 1 lone pair of electrons.
Hence, the correct answer is Option (1)
Example 4: The shape of a molecule is determined by
1)Valence bond theory
2)Molecular orbital theory
3) Valence shell electron pair repulsion theory
4)None
Solution
VSEPR (Valence Shell Electron Pair Repulsion) Theory - Valence shell electron-pair repulsion theory (VSEPR theory) enables us to predict the molecular structure, including approximate bond angles around a central atom of a molecule from the estimation of the number of bonds and lone pairs of electrons in its Lewis structure. The shape of a molecule depends upon the number of valence shell electron pairs (bonded or non-bonded) around the central atom. These pairs of electrons in the valence shell repel one another since their electron clouds are negatively charged.
Hence, the answer is the option (3).
Example 5: The reason for the change in bond angle in the different molecules having the same hybridization is given by:
1)Molecular orbital theory
2) Valence shell electron pair repulsion theory.
3)Valence bond theory
4)None
Solution
1. Electron pairs are always repelling each other and try to remain far apart
2. Different electron pairs have different orders of repulsion: L.P-L.P > L.P-B.P > B.P-B.P
3. If lone pairs are not present then geometry is equal to shape else geometry is not equal to the shape.
This all is explained by V.S.E.P.R. theory.
Hence, the answer is the option (2).
Example 6:
Which among the following molecules is (a) involved in sp3d hybridization, (b) has different bond lengths and (c) has lone pair of electrons on the central atom ?
[JEE Main 2025]
1) PF5
2) XeF4
3) SF4
4) XeF2
Solution:

(a) Hybridisation
(b) All bonds are not identical
(c) No lone pair on central atom

(a)
(b) 2 lone pairs on central atom

(a) Hybridisation
(b) All bonds are not identical
(c) 1 lone pair on central atom

(a) Hybridisation
(b) All bonds are identical
(c) 3 lone pairs on the central atom
From all the given molecules, SF4 molecule is involved in sp3d hybridization, has different bond lengths, and has a lone pair of electrons on the central atom
Hence, the correct answer is option (3).
Summary
The Valence Shell Electron Pair Repulsion is one of the primary models used in computing the geometry of molecules, given electron pair repulsion. It was developed by Gillespie and Nyholm. The theory postulates that electron pairs around a central atom arrange themselves as far away from each other as possible, thereby minimizing the repulsion and thus determining the shape of the molecule. The theory describes all geometries of the molecules by considering both bonding and lone pairs of electrons.
Also Check-
- NCERT Exemplar Class 11th Chemistry Solutions
- NCERT Exemplar Class 12th Chemistry Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Chemistry Notes:
