Zeroth Law Of Thermodynamics
The Zeroth Law of Thermodynamics is among the fundamental laws that establish the concept of temperature and thermal equilibrium: if two systems are each in thermal equilibrium with a third system, then they are in thermal equilibrium with each other. It provides a physical basis for the measurement of temperature because it proves that temperature is both a universal and transitive property. In essence, it gives a way to define and compare temperatures across different systems.It says that systems not in thermal equilibrium will interchange heat until they are at the same temperature. The Zeroth Law defines temperature in a clear and self-consistent way; hence, all subsequent laws and principles of thermodynamics need this to establish a firm base for thermal science.
This Story also Contains
- The Zeroth Law of Thermodynamics
- Some Solved Examples
- Summary
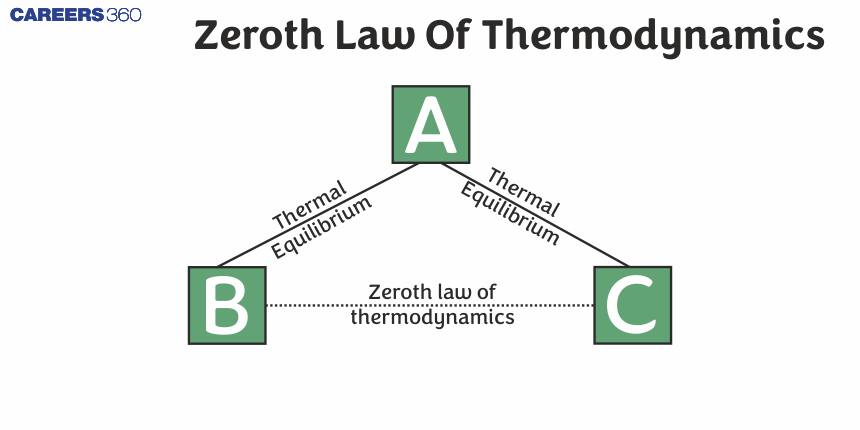
The Zeroth Law of Thermodynamics
If two systems are in thermal equilibrium with a third system, they must be in thermal equilibrium with each other.
If system B is in thermal equilibrium with system A and system C is also in thermal equilibrium with system A, system B, and system C are in thermal equilibrium with each other.

A hot body has a higher temperature than a cold body. The temperature of two bodies decides the direction of heat flow when the two bodies are put in contact. The heat flows from the hot body to the cold body till the bodies are at the same temperature it is known as thermal equilibrium.
Some Solved Examples
Example 1: Which of the following is/are chosen as the standard thermometric substance?
1) Gas
2)Liquid
3)Solid
4)All of the above
Solution
In gases, the smallest variation of change in temperature or pressure is recorded hence most gases are used as a standard thermometric substance.
Hence, the answer is the option (1)
Example 2: Which law states, “if two objects are in thermodynamic equilibrium with a third object, then they must be in thermodynamic equilibrium with each other”.
1)1st law of Thermodynamics
2)2nd law of Thermodynamics
3)3rd law of Thermodynamics
4) Zeroth Law of Thermodynamics
Solution
Zeroth law of thermodynamics states this fact.
Hence, the answer is the option (4).
Example 3: Which law of thermodynamics allows us to define the concept of temperature scale:
1)First law of thermodynamics
2)Second law of thermodynamics
3) Zeroth law of thermodynamics
4)Third law of thermodynamics
Solution
The zeroth law is incredibly important as it allows us to define the concept of a temperature scale. If two systems are each in thermal equilibrium with a third, they are also in thermal equilibrium with each other.
Hence, the answer is the option (3).
Summary
The Zeroth Law of Thermodynamics states that, provided system A is in thermal equilibrium with system B and if B is in thermal equilibrium with C, then A will be in thermal equilibrium with C. This law is of paramount importance in defining the temperature as something measurable and comparable. It allows the use of thermometers to measure temperature, ensuring that temperature readings are consistent and meaningful for different systems. The zeroth law provides the basis upon which the construction of temperature scales and calibration of thermometers can be built. It postulates that temperature is transitive: if two systems are separately in thermal equilibrium with a third system, they are also in thermal equilibrium with each other. This is a very major law in the process of heat transfer. It says that systems not in thermal equilibrium will interchange heat until they are at the same temperature. The Zeroth Law defines temperature in a clear and self-consistent way; hence, all subsequent laws and principles of thermodynamics need this to establish a firm base for thermal science.
