CuSO_{4} Full Form
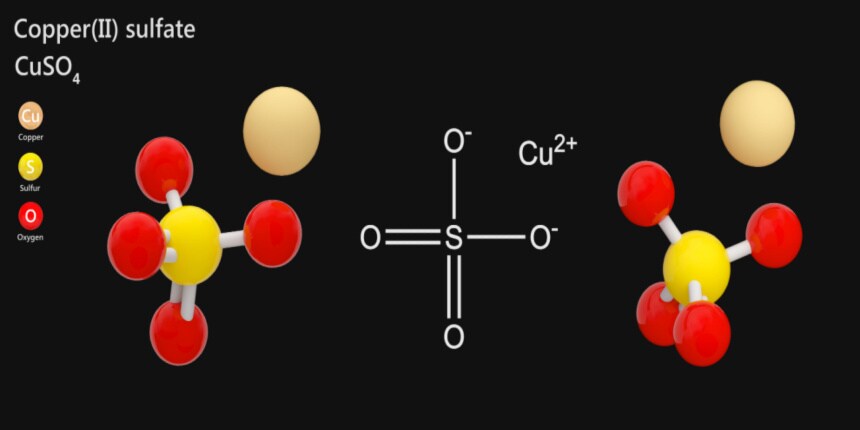
What is full form of CuSO_{4} ?
CuSO_{4} is an inorganic chemical compound, and it is known as Cupric Sulphate(CuSO_{4}) and Cuprous Sulphate(Cu_{2}SO_{4}). It is commonly called Copper Sulphate. This salt is produced by treating cupric oxide and sulphuric acid together, and it forms blue crystals. The most common form of copper sulphate is Pentahydrate which contains 5 molecules of water (CuSO_{4}.5H_{2}O). Pentahydrate was also known as blue vitriol, bluestone, the vitriol of copper, and Roman vitriol. Copper cation(Cu^{2+}) and sulphate anion((SO_{4})^{2-}) have an ionic bond between them.
Properties of CuSO_{4} :
Different forms of copper sulphate have different molar masses. The anhydrous form is around 169.60g per mole, whereas the pentahydrate form is around 249.685 g per mole.
The colour of the compound also varies with its forms. The Anhydrous form has grey-white colour, whereas the pentahydrate form has a bright blue colour.
The anhydrous form has a density of 3.6 g per cubic cm, and the pentahydrous has a density of 2.28 g per cubic cm.
The copper ions of copper sulphate react with chloride ions of HCl acid and form tetrachlorocuprate(II).
Cu^{2+} + 4Cl^{-} \rightarrow ( CuCl_{4})^{2-}
![]()
Copper Sulphate is highly soluble in water.