Elements Full Form
All substances around us are made up of atoms, which are further divisible into electrons, protons and neutrons. Substances containing only one atom are known as elements. They are pure substances, which cannot further be broken down chemically to form new substances. An element is described with respect to its number of protons, also called the atomic number.
- Atoms
- Molecules
- Atomic Number
- The Modern Periodic Table
- Properties
- Matter
- States of Matter
- Elements And Compounds
Atoms
The constituent of all matter are atoms. Initially thought of as indivisible by Dalton, an atom was later discovered to be constituted of sub-atomic particles like electrons, protons and neutrons. Each atom is a pure substance, an element. Each atom constitutes a fixed number of protons in its nucleus which defines it. For example, an Oxygen atom contains 8 protons. No other atom can contain 8 protons. However, an atom containing 8 protons may contain a different number of neutrons. This gives rise to isotopes, i.e., elements having equal numbers of protons but different numbers of neutrons.
Molecules
A molecule is a species constituted of multiple atoms joined together by either covalent or coordinate bonds. However, a molecule of an ionic substance does not exist.
A molecule may be constituted of either the same type of atoms or different types of atoms. This leads to two types of molecules- homonuclear (which contain the same type of atom) molecules like O2, H2, etc., and heteronuclear (which contain more than one type of atom) molecules like H2O, CO2, etc. All homonuclear molecules are elements whereas all heteronuclear molecules are compounds.
Atomic Number
Each atom is described and recognized with respect to its atomic number (Z), i.e., its number of protons. No two different types of atoms can have the same number of protons. All atomic elements are defined by their atomic number. It is the atomic number which finally helped in the classification and led to the formation of the modern periodic table.
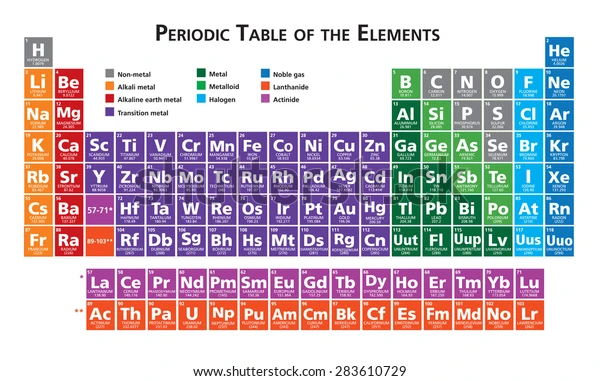
The Modern Periodic Table
After several failed attempts, the modern periodic table was made based on the arrangement of elements in ascending order of their atomic numbers. It follows the law that states “An element's atomic number determines both its physical and chemical properties.” This is because the only reacting electron(s) in an atom is/are it valence electron(s) and thus, all elements having the same number of valence electrons tend to follow a specific trend. Therefore, atomic number was the most suitable method for grouping elements in the periodic table.
Properties
Elements are pure substances. They may be atoms or molecules. Irrespective of the form they possess, they have some specific defined properties. These may be of two types- physical and chemical.
A property of matter that is unrelated to the change in its chemical makeup is called a physical property. Any measurable characteristic whose value characterises the state of a physical system is included in this. The transitions between a system's current states can be described by changes in the physical characteristics of the system. A few examples of these include colour, hardness, texture, solubility, density, melting point, malleability, boiling point and electrical conductivity.
Any quality that can only be formed by altering a substance's chemical identity is referred to as a chemical property, and it can occur during or after a chemical reaction. These explain how the makeup of a substance can cause it to undergo a chemical change or reaction. The matter has the capacity to undergo chemical change due to the elements, electrons, and bonds that it is composed of. A few examples of these include chemical stability, flammability, toxicity, heat of combustion, pH level, and rate of radioactive decay.
Matter
According to general chemistry, anything that occupies space and has mass is termed as matter. These include the general objects around us, like the chair, table, window, air, birds, etc. However, the constituents of all matter are atoms. Elements themselves are made up of thousands of millions of atoms. Each atom is arranged in a fixed manner to produce crystalline substances, having defined lattices. These elements further combine together to form compounds. Elements or compounds, mix together to form mixtures, without reacting chemically.
States of Matter
Matter exists in multiple forms. Each of these ‘forms’ is known as a state or phase of matter. The states are defined by the temperature, volume and pressure applied to the particles. These factors in turn decide the energy of the constituent particles and their ability to move. The commonly known states of matter include the following:
Solid: In this state, the constituent particles have the least amount of Kinetic energy and are compactly packed, restricting their movement. The particles have fixed positions and they vibrate in their place. As a result, the substance in this state has a fixed shape and volume.
Liquid: In this state, the constituent particles have some kinetic energy and are not fixed. Also, their movement is not completely restricted and they can flow. Thus these come under “fluids”. They have a fixed volume but are able to take the shape of any container they occupy.
Gases: In this state, the constituent particles have abundant kinetic energy and they are not fixed. Their movement is completely unrestricted and thus they are also able to flow. These also fall under fluids. They neither have a fixed shape nor volume nor tend to occupy the shape and volume of the container that they occupy.
Elements And Compounds
Similarities
They are both chemical substances
They are both made up of atoms
None of them is a mixture, but a pure substance
They cannot further be broken down by physical means
Differences
Criteria | Elements | Compounds |
Definition | A chemical element is a species of atoms, including the pure substance made entirely of that species, that have a specific number of protons in their nuclei. | A chemical compound is a substance that contains atoms from multiple chemical elements held together by chemical bonds. It is made up of numerous identical molecules. |
Chemical composition | They are made up of same types of atoms | They are made up of different types of atoms |
Structure | These may exist as molecules or atoms but never as ions | These exist only as molecules or ionic compounds |
Bonding | If molecules, only covalent or coordinate exist between atoms | The bonding here may be covalent, coordinate or ionic |
Examples | Carbon (C), Nitrogen (N), Nitrogen gas (N2), Hydrogen (H), Hydrogen gas (H2), etc. | Sodium chloride (NaCl) is an ionic compound, Water (H2O) is a covalent molecule, Carbon monoxide (CO) has a coordinate bond, etc. |
Frequently Asked Questions (FAQs)
The states of matter are the physical forms in which matter exists. Around 22 states have been discovered up till now, however, the main four states of matter known are- solid, liquid, gas and plasma. Some other forms include Bose-Einstein condensate, excitonium, photonic matter, ionised plasma, quark-gluon plasma, and fermionic condensate.
Pure Substances | Mixtures |
A pure substance is a type of matter with a defined chemical makeup and physical characteristics. | A mixture is a substance made up of two or more dissimilar chemical compounds that are physically put together. |
It has chemical bonds between. | It has no chemical linkage. |
It may be an element or a compound. | It may be heterogeneous or homogeneous. |
Examples include: Glucose, Carbon dioxide, Hydrogen gas, etc. | Examples include: Salt water, sugar syrup, etc. |
The periodic table lists elements discovered or prepared in the laboratory in the order of their increasing atomic numbers. This enables to us place the elements under appropriate groups and thus predict the possible values of physical (like boiling point, melting point, etc) and chemical (valence electrons, possible bonds, etc) quantities. Currently, there are about 120 known elements. Some of these are artificially prepared in the laboratory and some are extremely reacting, hardly being able to exist under normal conditions.
The periodic table lists about 120 known elements, naturally discovered or artificially prepared. Each of these elements falls under one of the 18 groups of the table. However, these elements may even be broadly divided into categories like metals, nonmetals and metalloids.
A metal is a substance that exhibits a shiny appearance when freshly processed, polished, or shattered, conducts electricity and heat rather well and usually has a very high oxidation tendency. Examples include Sodium (Na), Iron (Fe), etc.
A nonmetal is a chemical element that typically doesn't have a lot of metallic characteristics; examples include colourless vapours and glossy solids. They are usually bad conductors of heat and electricity and also have a high reduction potential. Examples include Chlorine (Cl), Carbon (C), etc.
A type of chemical element known as a metalloid has a majority of properties that fall between those of metals and nonmetals, or that are a combination of both. Examples include Antimony (Sb), Arsenic (As), etc.
The atomic number of chlorine is 17.
The atomic number of calcium is 20.
The atomic number of oxygen element is 8.