FADH full form
What is the full form of FADH?
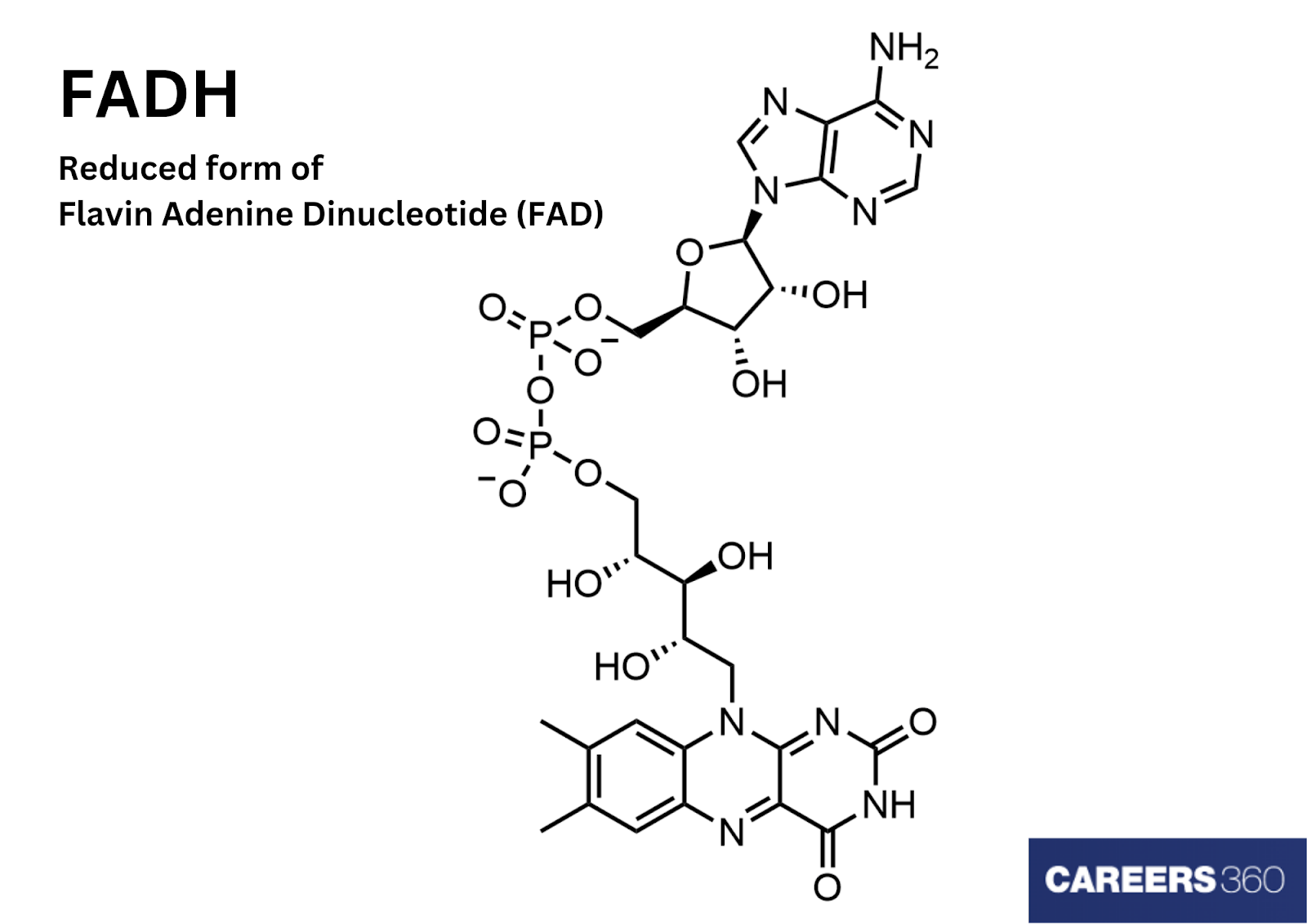
FADH - Flavin adenine dinucleotide hydride is one of the forms of FAD (Flavin adenine dinucleotide). FAD is a redox-active coenzyme. It gets associated with various proteins and performs enzymatic actions in metabolism. FADH can be formed by either the reduction of FAD or the oxidation of FADH2. It is also the resultant product of various metabolic activities.
- What is the full form of FADH?
- Forms of FAD
- Chemical formation
- Properties of FADH
- Functions and clinical significance

Forms of FAD
FAD exists in four redox states: flavin-N(5)-oxide, quinone, semiquinone, and hydroquinone. FAD can be interchanged into these forms by accepting and donating electrons. FADH2 is the hydroquinone form of FAD, formed by accepting two electrons and two protons from its quinone (fully oxidised) form.
While the semiquinone form, i.e., FADH, can be formed by accepting or donating one electron or proton, respectively. Flavin-N(5)-oxide is the super oxidised form of the FAD enzyme. FAD undergoes different chemical mechanisms to form its different forms.
Chemical formation
Adenosine Monophosphate and Flavin Mononucleotide (FMN) are joined by their phosphate groups to form flavin adenine dinucleotide (FAD). Adenine is a nitrogenous base. At 1' carbon, it binds with cyclic ribose sugar, which then binds with a phosphate group at 5' carbon. Thus, adenosine monophosphate is formed.
Flavin mononucleotide is produced by riboflavin bonded with a ribose sugar and a phosphate group. FAD, on reduction, forms FADH2 by adding 2H+ and 2e-. While the loss of 1H+ and 1E- results in the formation of FADH. FADH can be converted back to FAD by losing another H+ and e-.
Properties of FADH
In the aqueous solution, the molecules exhibit different colours due to the oxidative nature of flavin. Flavin-N(5)-oxide is yellow-orange, FAD is yellow, FADH is blue-red, and FADH2 is colourless in the aqueous solution.
Different forms of FAD have different properties.FAD, for example, has an aromatic ring, whereas FADH2 does not. This is why FADH2 is a highly energy-carrying molecule stabilised by oxidation, which forms FAD again. The half-reduced form, FADH, is unstable in an aqueous solution.
FAD and its different forms can be studied using UV-Vis and fluorescence spectroscopies. The oxidative properties of all four forms of FAD can be studied using different absorption spectra.
Functions and clinical significance
Due to its positive reduction potential, it is a highly potent oxidising agent. Thus, cells utilise FAD in the oxidation reactions and play a vital role in metabolic reactions such as electron transport, DNA repair, amino acid catabolism, citric acid cycle, etc.
FAD and its forms, such as FADH and FADH2, are used in the pharmaceutical sector to create supplement diets for certain diseases. In the agricultural industry also, it is used as a supplement for animals and food colourants.
Since FAD is an essential part of metabolic activities in the body, scientists are using FAD to investigate the effect of different drugs on pathogens such as bacteria. Since FAD has fluorescence properties, it is used to detect the early signs of oral cancer. It is also used as fluorescence assistance in biopsy.
Frequently Asked Questions (FAQs)
FADH is the semiquinone form of FAD.
Riboflavin, also called vitamin B2, is an essential vitamin for our body.
FADH plays a significant role in metabolism by accepting or losing protons and electrons to form FAD and FADH.
The maximum absorbance of FAD can be observed at a wavelength of 450 nm.
FADH can be formed by utilising electrons removed from acetyl CoA during the reaction mechanism of the citric acid cycle.