FeSO4 Full Form
What is the full form of FeSO4?
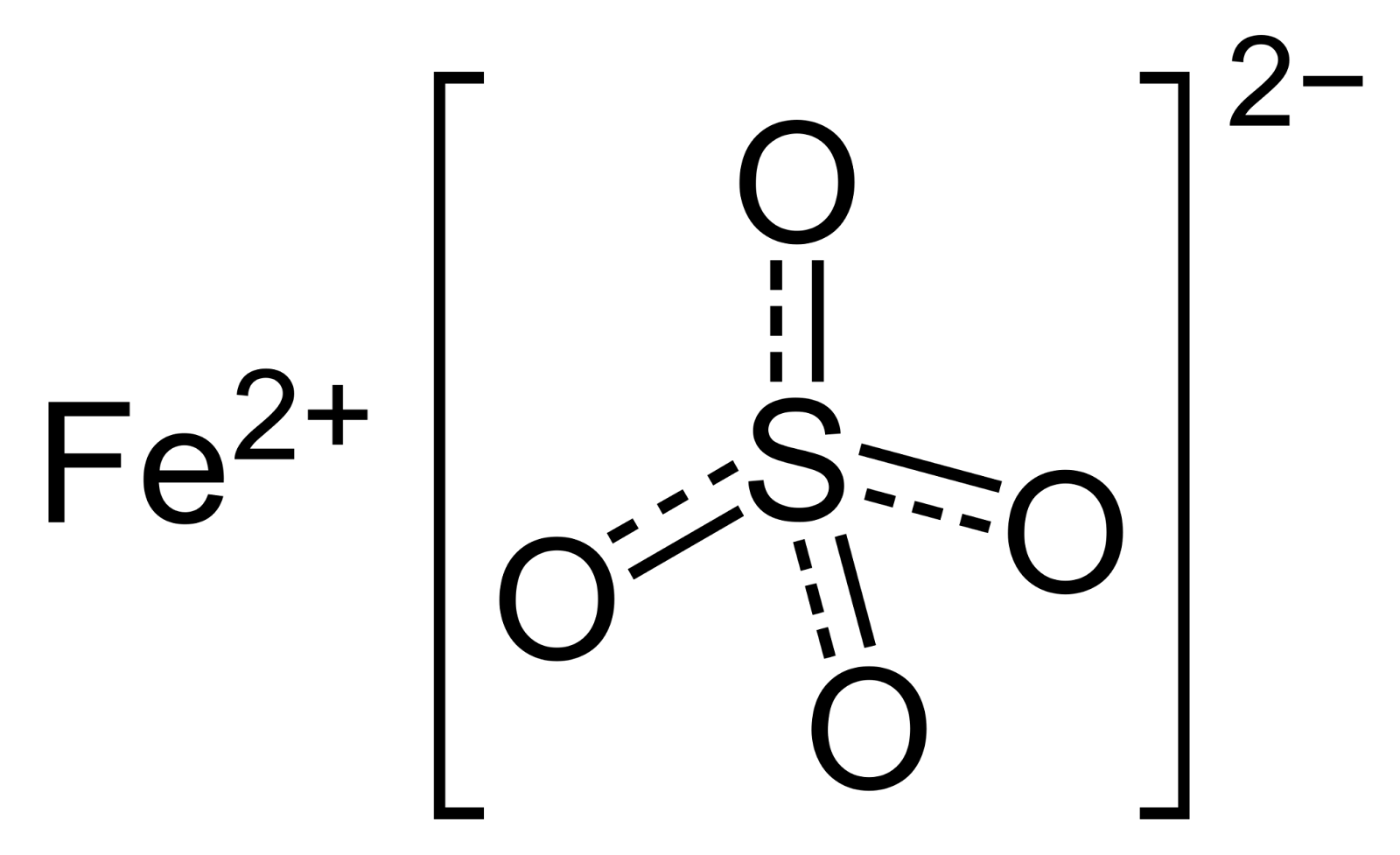
Iron(II) sulphate or ferrous sulphate indicates a range of salts having the formula Fe SO₄·xH₂O. These compounds exist most frequently as the heptahydrate but various values for x are known. The elements of FeSO4 are iron, sulphur and oxygen. FeSO4 is an ionic compound.
In this article, we will know about FeSO4 in detail.
About The FeSO4
Ferrous sulphate is a chemical compound having the formula FeSO4.From ancient times it is known as copperas and as green vitriol, the most common form of this material is the blue-green heptahydrate.
Physical Properties of Ferrous Sulphate (FeSO4)
Formula: FeSO4
Molecular weight:151.908 g/mol
Appearance: A set of salts with the formula FeSO4 xH2O is referred to as ferrous sulphate. Other names for iron(II) sulphate include ferrous sulphate, copperas, melanterite, iron vitriol, and green vitriol. The blue-green heptahydrate (a hydrate with "7" water molecules) is the most prevalent variant.
Odour: Odourless
Solubility: Soluble in water
Melting point: 60-64°c
pH: Acidic
Chemical Properties of Ferrous Sulphate (FeSO4)
Aluminium sulphate and metallic iron are obtained when ferrous sulphate interacts with aluminium in a displacement reaction. The reaction is described as below.
\begin{equation}
2 \mathrm{Al} +3 \mathrm{FeSO} 4 \rightarrow \mathrm{Al} 2(\mathrm{SO} 4) 3+3 \mathrm{Fe} \end{equation}
![]()
In the presence of sulfuric acid, ferrous sulphate interacts with potassium permanganate to produce ferric sulphate, manganese sulphate, potassium sulphate, and water.
\begin{equation}
10 \mathrm{FeSO} _{4}+2 \mathrm{KMnO}_{4}+8 \mathrm{H}_{2} \mathrm{SO}_{4}\rightarrow 5 \mathrm{Fe} _{2}(\mathrm{SO} _{4})_{3}+2 \mathrm{MnSO}_{4}+8 \mathrm{H} _{2} \mathrm{O}+\mathrm{K} _{2} \mathrm{SO} _{4}
\end{equation}
Removal of Oxide Layer From FeSO4 using Pickle Liquor
1 .The material is submerged in a tank of "pickle liquor" to remove this oxide coating. Pickle liquor can take many different shapes. In hydrochloric or sulfuric acid, carbon steels with an alloy concentration of less than 6% are frequently pickled. A two-step pickling procedure is necessary for steels with a greater carbon content, and additional acids (such as phosphoric, hydrofluoric, and nitric acid) are utilised.
2. We are going to concentrate on the hydrochloric acid pickling of steel for the production of ferrous sulphate. Ferrous sulphate is produced as a byproduct of the pickling process, which involves combining hydrochloric acid with iron-based steel.
Applications of Ferrous Sulphate (FeSO4)
Ferrous sulphate is used to fortify foods with iron.
Ferrous sulphate can be used to treat an iron deficiency known as anaemia.
Ferrous sulphate has been used in the textile industry for centuries as a dye fixative.
Ferrous sulphate is used historically to blacken leather and as a constituent of ink.
Ferrous sulphate is a useful reagent in the identification of mushrooms.
Ferrous sulphate has been used in the purification of water through the process of flocculation and for the purpose of removal of phosphate in the treatment of municipal and industrial sewage.
Ferrous sulphate is used to stain concentrate and limestones.
Important reaction of FeSO4
Decomposition Reaction of FeSO4
Crystals of ferrous sulphate include molecules of water (FeSO4. 7H2O). Anhydrous ferrous sulphate (FeSO4) is produced when ferrous sulphate crystals are heated and lose their water content. As a result, they turn from bright green to white.
Anhydrous ferrous sulphate breaks down into ferric oxide (Fe2O3), sulphur dioxide (SO2), and sulphur trioxide with additional heating (SO3). Thus, the gas released has a sulfuric burning smell.
\begin{equation}
2 \mathrm{FeSO} 4(s) \rightarrow \mathrm{Fe} 2\mathrm{O} 3 (s)+ \mathrm{S}\mathrm{O} 2 (g)+ + \mathrm{S}\mathrm{O} 3(g) \end{equation}
![]()
Frequently Asked Questions (FAQs)
FeSO4 is not used in titration because it gets readily oxidised.
Yes, FeSO4 can be stored in an aluminium container.
When FeSO4 is exposed to air, it is rapidly oxidised and gets coated with brownish-yellow ferric sulphate.
FeSO4 is a weak acid and a strong base.
On heating the colour of FeSO4 changes from light green to white.
