H2SO4 Full Form
What is the full form of H2SO4?
H2SO4 is the chemical formula for Sulphuric Acid, also known as Oil of Vitriol, which is a highly corrosive, strong mineral acid that is a colourless to slightly yellowish, odourless, oily liquid. It is a strong acid, with a pK value of “-3”. It is mainly used in the production of fertilisers, dyes, and detergents, as well as in the chemical industry.
- What is the full form of H2SO4?
- Structue of H2SO4
- Uses of H2SO4
- History of H2SO4

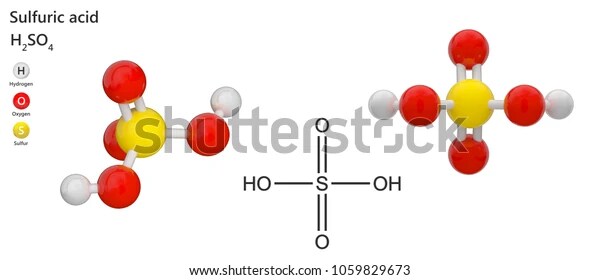
Sulphuric acid is a very strong acid, composed of two hydrogen atoms, one sulphur atom, and four oxygen atoms. It has a molecular weight of 98.08 g/mol and a molar mass of 98.08 g/mol. It is highly soluble in water, with a solubility of 1.84 g/100 mL at 25 °C. It has a density of 1.832 g/cm3, a boiling point of 337.1 °C, and a melting point of 10.4 °C.
Sulphuric acid is produced in large quantities and used in many industries, including fertilisers, dyes, detergents, and other chemicals. It is also used in the manufacture of batteries, in the pickling of steel, and in the purification of petroleum products. It is also used as a strong oxidising agent, as a dehydrating agent, and as a catalyst in many chemical reactions.
Structue of H2SO4
Uses of H2SO4
While sulphuric acid is highly corrosive and dangerous if mishandled, it is an essential chemical for many industrial processes.
In the automotive industry, sulphuric acid is used in the production of lead-acid batteries, which are used in cars, motorcycles, and other vehicles. The acid is used to create a chemical reaction that causes the lead and other metals to form a paste-like substance. This paste is then used to form the battery’s cells, which store and provide energy to the vehicle.
In the textile industry, sulphuric acid is used to produce synthetic fabrics such as polyester, nylon, and spandex. The acid is used to dissolve and break down the polymers in the fabric, which helps to create a uniform consistency and colour. It is also used to create dyes and pigments for fabrics.
In the construction industry, sulphuric acid is used to etch or corrode concrete and other materials. This process is often used to create decorative patterns and make surfaces more resistant to wear and tear. It also cleans and removes rust and other contaminants from surfaces.
In the food and beverage industry, sulphuric acid is used as an additive to preserve food and prolong its shelf life. It is also used to help break down the proteins and carbohydrates in the food, which helps to improve its flavour and texture. Furthermore, it is used as a disinfectant, as it helps to kill bacteria and other microorganisms.
In the medical industry, sulphuric acid is used to manufacture drugs and medicines. It is also used to create sterile solutions and to clean and disinfect medical equipment.
Finally, sulphuric acid is also used in the production of fertilisers and other agricultural products. It is used to break down minerals and other nutrients, which can then be used to help plants and crops grow.
In conclusion, sulphuric acid is essential for many industries and processes. It is used for a wide variety of purposes, from cleaning and disinfecting to producing drugs and medicines. Despite its corrosive nature, it is an incredibly useful and versatile chemical that is necessary for the advancement of many industries.
History of H2SO4
The history of H2SO4, or sulphuric acid, can be traced back to the ancient Greeks. In the 5th century BCE, the Greek philosopher and scientist Theophrastus wrote of a substance called "oil of vitriol, " created by burning green vitriol. This oil of vitriol was likely the first form of sulphuric acid ever created.
In the Middle Ages, sulphuric acid was used for a variety of purposes, including bleaching fabrics, dyeing cloth, and as a cleaning agent. In the 17th century, Johann Glauber, a German chemist, used sulphuric acid to produce sodium sulphate which was used in the making of glass and the production of certain types of paper.
In the 18th century, sulphuric acid was used in the production of alum and vitriol, two chemicals that were used in the making of various products.
In the 19th century, the industrial revolution saw a huge increase in the use of sulphuric acid due to its high reactivity and ability to be used in various reactions. It was used in the production of dyes, fertilisers, and explosives.
In the 20th century, sulphuric acid was used in a variety of industries, including oil refining, chemical manufacturing, pharmaceuticals, and metal processing. In the early part of the 20th century, the use of sulphuric acid increased exponentially, making it one of the most produced chemicals in the world.
Today, sulphuric acid is used in a variety of industries, including paper manufacturing, oil refining, wastewater treatment, and chemical manufacturing. It can also be used in the production of fertiliser, batteries, and detergents.
Frequently Asked Questions (FAQs)
H2SO4 is corrosive and can be hazardous to health if not handled properly. It can cause skin and eye irritation, and prolonged exposure can cause more serious health problems such as respiratory irritation and damage to the throat, lungs, and other organs. Inhalation of sulphuric acid fumes can lead to coughing, choking, and even death. It is important to take safety precautions when working with H2SO4, including wearing protective clothing, safety goggles, and gloves.
H2SO4 should be stored in a cool, dry place away from sunlight and other sources of heat. It should also be kept away from combustible materials and any open flames. It should be stored in a tightly sealed container and labelled clearly. It should never be stored near food or preparation areas, and any spills should be cleaned immediately.
H2SO4 is a strong acid, meaning it has a high level of acidity. Its pH is usually between 0 and 1. It is a colourless liquid that is highly soluble in water and other polar solvents. It is non-flammable and non-volatile. It has a strong Odour and can cause irritation and burns if it comes into contact with the skin or eyes. It is hygroscopic, meaning it absorbs moisture from the atmosphere.
When handling H2SO4, it is important to wear protective clothing, safety glasses, and gloves. It should also be stored in a cool, dry place and away from any sources of heat or ignition. If there is any contact with skin or eyes, the affected area should be washed immediately with plenty of water.
The molarity of 18M H2SO4 is approximately 18M. This means that there are 18 moles of H2SO4 per litre of solution.