HCHO Full Form
What is the full form of HCHO?
HCHO or CH2O is the chemical formula for Formaldehyde (systematic name methanal). It is a naturally occurring organic compound. It is the simplest of the Aldehydes and is used in various chemical manufacturing processes in a large variety. It is a highly volatile organic compound and belongs to the group of most dangerous compounds present in the air.
- What is the full form of HCHO?
- Formaldehyde
- Properties
- Origins Of Formaldehyde
- Production Of Formaldehyde
- Applications Of Formaldehyde
- Formaldehyde As A Pollutant
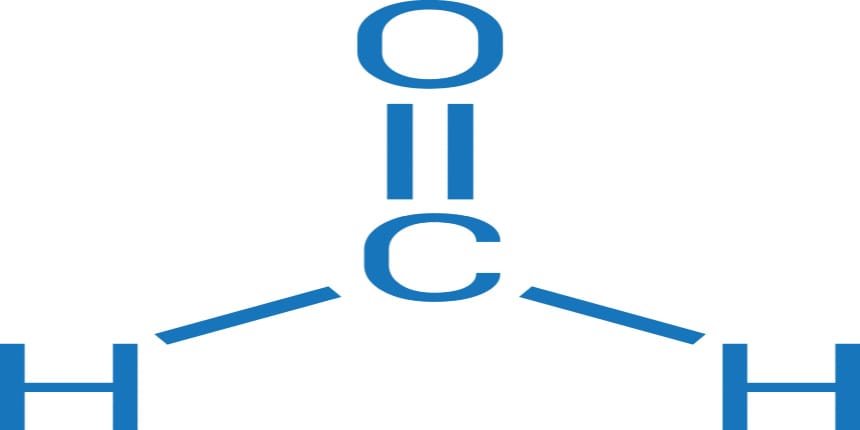
Formaldehyde
The chemical formula for Formaldehyde is structured HCHO. The pure compound is invisible to the eyes as it is colourless but has a very peculiar pungent odour that is extremely irritating to the mucous membrane. It is highly Carcinogenic in humans and is associated with several types of cancer in humans and other animals. It is chiefly present in tobacco products but is formed by burning additives in it.
Structure:
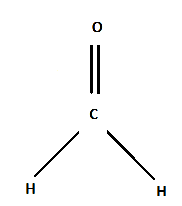
One carbon atom, two hydrogen atoms, and one oxygen atom comprise the chemical compound formaldehyde. In order to complete its valency, a single Carbon atom forms a double bond with an Oxygen atom and a single bond with each of the two hydrogen atoms.
The carbonyl functional group's polar bond with oxygen makes the substance extremely reactive. The ability of the oxygen atom in the carbonyl group to attract electrons is greater than that of the carbon atom. At both ends of the functional group, this results in a partial negative charge and a partial positive charge. As a result, it adheres to the polar particles and gains some ability to draw and give electrons.
Properties
At room temperature, formaldehyde is a flammable, colourless gas with a strong, suffocating odour. Due to its many favourable chemical reactions and ease of polymerization, formaldehyde is one of the most significant industrial chemicals in the world.
Physical Properties
815 kg/m3 for density
Molar Mass/Molecular Weight - 30.031 g/mol
-19 °C is the Boiling Point
-92.0 °C is the melting point
It has a Suffocating odour appearance.
Uncoloured in gaseous as well as in liquid form.
The elements (Carbon, Hydrogen, Oxygen) are Covalently bonded together.
Soluble in polar solvents like acetone and water.
Chemical Properties
Formaldehyde reacts with a base like sodium hydroxide and forms sodium formate and methanol. The chemical equation is given below.
\2HCHO + NaOH \rightarrow HCOONa + CH_3OH
![]()
Formaldehyde reacts with ammonia to form hexamethylenetetramine and water. The chemical equation is given below.
6 \mathrm{HCHO}+4 \mathrm{NH}_3 \rightarrow\left(\mathrm{CH}_2\right)_6 \mathrm{N}_4+6 \mathrm{H}_2 \mathrm{O}
![]()
Origins Of Formaldehyde
Processes in the upper atmosphere contribute to the production of natural Formaldehydes up to 90%. The origin of Formaldehyde in an area depends on various factors that include; air pressure, temperature, and humidity.
The Man originated sources of Formaldehyde are:
Construction Material
Carpet
Textiles such as curtains, sofa covers, etc.
Wood panels
Carpeting
Furniture made of fibre- or chipboard such as chairs, closets, tables, kitchens, nightstands, beds, etc.
Wallpaper
Paint
Indoor sources rich in Formaldehyde content:
Cleaning products
Cooking
Lighting candles or incense
Smoking
Air fresheners
Various cosmetics
Production Of Formaldehyde
Industrial production of formaldehyde involves the catalytic oxidation of methanol. Silver metal, iron(III) oxide, iron-molybdenum oxides with a molybdenum-enriched surface, or vanadium oxides are the most popular catalysts. According to the chemical equation, formaldehyde is produced when methanol and oxygen react at about 250 to 400 °C in the presence of iron oxide along with molybdenum and/or vanadium:
\2 CH_3OH + O_2 \rightarrow 2 CH_2O + 2 H_2O
![]()
The average operating temperature for the silver-based catalyst is around 650 °C. Formaldehyde is produced on it simultaneously by the two chemical reactions depicted above and the dehydrogenation reaction.
CH_3OH \rightarrow CH_2O + H_2
![]()
Although the oxidation of methane could theoretically produce formaldehyde, this method is not practical for industrial use because methanol is easier to oxidise than methane.
Applications Of Formaldehyde
Medical purpose: Formaldehyde is yield in producing "Methenamine '', which is preferred as a urinary antiseptic.
Industrial purposes: Formaldehyde is used to produce explosives like cyclonite or RDX (Royal Demolition eXplosive) and Pentaerythritol tetranitrate (PENT). A large quantity of Formaldehyde is used in the production of Urea-Formaldehyde resin, Phenol-Formaldehyde Resin, and Acetal resin (polyoxymethylene).
It is used as a Disinfectant and Embalming agent, also a soil sterilant.
Formalin, an aqueous product of Formaldehyde, is preferred in laboratories as a specimen preservative.
To improve the yield of fuel, it is used in Petroleum and Natural gas Industries.
Formaldehyde As A Pollutant
In 2011 the United States National Toxicology Program marked Formaldehyde as "known to be a human carcinogen" as it poses a significant danger to human health. It is a common indoor air pollutant. In a gaseous state, it can affect the eyes, throat, and lungs or even trigger Asthma even at low concentrations. Prolonged or chronic exposure to Formaldehyde can result in cancer. In ambient air Formaldehyde usually breaks down quickly to create Formic acid and Carbon monoxide which are very harmful to humans.
