IUCD Full Form
What is the full form of IUCD?
An IUCD stands for Intrauterine Contraceptive Device. An IUCD is a small device that rests inside the uterine part of the female body to prevent pregnancy. It is made from plastic and copper and is also termed a Cu-IUCD or coil. The IUCD is attached to two nylon threads that pass through the neck of the cervical region of the uterus to lie in the vagina. The threads allow the IUCD to be withdrawn quickly.
- What is the full form of IUCD?
- Types of IUCD
- Working of IUCD
- Effectiveness of IUCD
- Insertion of IUCD
- Removal of IUCD
- Advantages of IUCD
- Disadvantages of IUCD
- Emergency Condition
- Precautions of IUCD
- Alternatives of IUCD
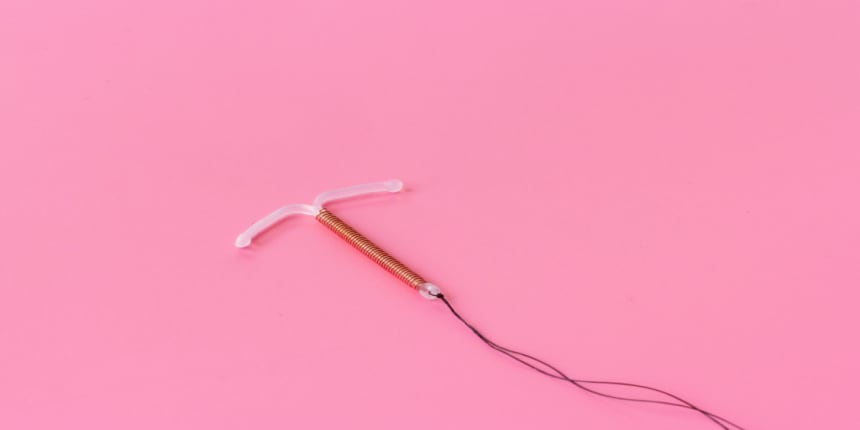
Most of the IUCD devices are T-shaped and as long as a matchstick. Therefore, the device gets inserted neatly inside the uterus as it is only a little longer than a matchstick. An IUCD is a safe, reversible method of female contraception, meaning that it is removed after a period or as consulted by health professionals.
Types of IUCD
There are three different types of IUCD. These are:
Copper-bearing IUCDs: These devices are fabricated of plastic with copper sleeves and copper wire on the plastic, for example, TCu-380A and MLCu-375.
Hormone-releasing IUCDs: They are made of plastic and gradually release small amounts of progesterone or other progestin hormones, such as LNG-20 and Progestasert.
Inert or unmedicated IUCDs are made of plastic or stainless steel only, for example, Lippes Loop and Chinese stainless-steel rings.
Working of IUCD
The IUCD device primarily makes it difficult for sperm cells to fertilize an egg inside the uterus. This is an effect of the copper released by the device. The presence of copper makes the mucus at the neck of the cervix and the womb lining hostile to sperm and eggs. The device makes the uterine lining much less likely to accept an egg and prevents the sperm from getting inside the uterus through the cervix; thus, the sperm cannot travel through the womb.
The IUCD does not result in an abortion or end of pregnancy; it only prevents the eggs and sperm from meeting each other. Many copper IUCDs work well for five to ten years and are very effective for the entire duration with almost zero failure.
Effectiveness of IUCD
Using an IUCD device is safe and effective as female surgical sterilization in preventing pregnancy, but unlike sterilization, it is an entirely reversible contraceptive method. Less than 1% of women, 0.6 to 0.8 per 100 women using the Copper T 380A IUCD, become pregnant during the first year of use. Consequently, the calculated risk rate of pregnancy over the ten-year service life of an IUCD is less than 3%. Furthermore, fertility can be resumed soon after the removal of the device.
The Copper T 380A can prevent pregnancy in women for up to 12 years. However, its effectiveness is reduced after seven years of utilization, after which the probability of becoming pregnant increases. Some countries suggest replacing or removing it ten years from the projection date.
The critical point is that a woman's fertility returns immediately after removing the IUCD device.
Insertion of IUCD
An IUCD device can be inserted anytime after confirming that the person is not pregnant. A vaginal examination and checking of the uterus's size and position follow the process. After the test, the IUCD is fitted using a tiny plastic insertion device.
The professionals teach the patients how to feel the threads of the IUCD so they can check it is in place. It is suggested to check the threads once a month, just after a period.
Inserting the IUCD device can cause discomfort to some patients as there might be pain or vaginal bleeding after the insertion. Therefore, painkillers and medicines such as paracetamol or ibuprofen are suggested to ease the condition after the symptoms arise.
Removal of IUCD
If there is a possibility of pregnancy, one must consult with the doctor to get the IUCD device removed as soon as possible. There is an increased chance of miscarriage if the device is left in place during pregnancy.
The best time to remove the device is during the period. The professionals remove the IUCD device by pulling the threads. The removal process may cause pain in the abdomen for some time.
Advantages of IUCD
There are many advantages to using IUCDs. They are as follows-
No requirement to use other contraception after insertion of an IUCD.
The IUCD does not interfere during sexual intercourse or sex drive (libido).
It has zero side effects on the rest of the body as it is not a hormonal method.
It does not affect mood, weight, or libido as the usage of other contraceptive methods does.
It does not pose a threat to cancer in the future.
Disadvantages of IUCD
Using an IUCD device may cause certain complications that must be noted. They are-
It may cause an allergic reaction inside the uterus due to the presence of the equipment, materials, or taking medication.
Using the device may cause a vasovagal reaction.
The device strings may be lost or not felt at the top of the vagina.
There is a risk of losing the IUCD device as it is tiny.
There is a possibility that the device may make a hole inside the womb.
There is a risk of infection.
It may cause an increase in period pain and bleeding.
It may give rise to pregnancy problems in the future.
Emergency Condition
A person using an IUCD device should take care of certain emergency conditions. They should know when to consult with the doctor if symptoms like these occur:
Severe cramping in the abdominal region.
unusual vaginal bleeding
fever without another cause may be a sign of infection inside the uterus
bad-smelling discharge or clot from the vagina
delayed period and irritation
If one can feel the hard plastic of the device or if one can't feel the string must go to the doctor immediately
Precautions of IUCD
Certain precautionary measures must be taken while using or intending to use an IUCD device.
Women with the following conditions are advised not to use an IUCD device-
Pelvic inflammatory disease (PID): IUCDs can negatively affect pelvic infections as they may result in microbiological contamination during insertion. A woman having such issues must be advised to use another method.
Suspected pregnancy: IUCDs cause toxic effects on the fetus and may result in abortion in pregnant women. So, a suspected pregnant woman must not be recommended an IUCD device.
Irregular genital tract bleeding: This is a symptom of uterine cancers. Such cases should be referred to the hospital for further investigation before using an IUCD.
Allergy to any device element: The previous history of allergy to any medication or substance must not be recommended as an IUCD device. A different contraceptive method must be suggested in such cases.
Preventive measures are to be suggested by doctors before the use of IUCDs.
A woman must consult a doctor for further examination if she wishes to use an IUCD and gets diagnosed with heart disease or abnormalities of the uterus resulting in a distorted cavity or might have a history of ectopic pregnancy or a weakened immune response.
Any woman using an IUCD device must be advised that they can keep track of the device by checking for its strings, and they can get an idea about the threads by putting a finger into their vagina. It is certainly recommended that women check their IUCD devices after each period.
A shorter-than-normal string can be a warning sign of a misplaced IUCD. Missing strings may indicate the expulsion of the IUCD. When the string is missing, one should advise the woman to visit the professionals to have an alternative method of birth control. The patient may need to take a pregnancy test if the woman misses a period while on an IUCD.
Alternatives of IUCD
There are other non-permanent and permanent methods of female contraception other than IUCD. These are-
A levonorgestrel intrauterine system hormone implants
Oral contraceptive pill
The permanent method of female contraception is sterilization.
There is a non-permanent method of male contraception, such as using a condom. Still, there is a risk factor of failure as there may be manufacturing defects related to the material used to make condoms. One of the permanent methods of male contraception is vasectomy.
Frequently Asked Questions (FAQs)
Howard Tatum and Jamie Zipper introduced the copper intrauterine device in 1967.
Oral contraceptives or birth control pills are medications that contain a hormone that is taken by mouth to prevent pregnancy. They work by inhibiting ovulation and preventing sperm from penetrating through the cervix, thus preventing pregnancy. The most common oral contraceptives are monophasic pills.
Vasectomy or male sterilization is a process where the tubes that transport sperm from a man's testicles to the penis are nicked, blocked or sealed with heat. This prevents the sperm from mixing with the semen when they ejaculate and stops them from entering the uterus during intercourse. This prevents the fertilization process.
Using an IUCD is a personal choice, and they are a safe and effective way of preventing pregnancy. The device was not much popularized in India during the 50s, but after India attained Independence, the use of IUCD devices gained importance, followed by taking contraceptive pills and sterilization methods.
Medical professionals remain cautious about recommending IUCDs as there are instances that there were lawsuits filed against the maker of a hormonal IUCD. Thus, the IUCD also suffered negative PR due to many such instances.
Side effects after the insertion of the IUCD can include cramps and back pain which last only a few hours. Therefore, it is suggested to wait until the next day to resume physical activities or exercise. Furthermore, there is no medical objection to avoiding exercise after the insertion of the device.
There is a risk of expulsion of the device while using vibrating gym plates and thus advised to avoid the practice for a few weeks until they adjust to the change.
It is not known whether IUCDs increase the risk of acquiring HIV. The effect of IUCDs on the uterus and cervix may create an environment favourable for HIV transmission. There is the possibility that the intense bleeding associated with the use of IUCDs may increase the transmission of the virus from HIV-positive women to their partners.