KOH Full Form
Whatis the full form of KOH?
The full form of KOH is Potassium Hydroxide. It is a strong base of the alkali metal Potassium, which is represented by K. It is an inorganic compound and is commonly known as caustic potash. It is termed so since it reacts and damages any organic tissue it comes in contact with. This explains the term ‘caustic’. Alkali metals are highly reactive species and therefore, their electrolytes are strong bases that dissociate completely. KOH is found as orthorhombic or rhombic, deliquescent chunks, lumps, or sticks that are white, colourless, or slightly yellow and have a crystalline fracture. As a solution, however, it is transparent.
Structure
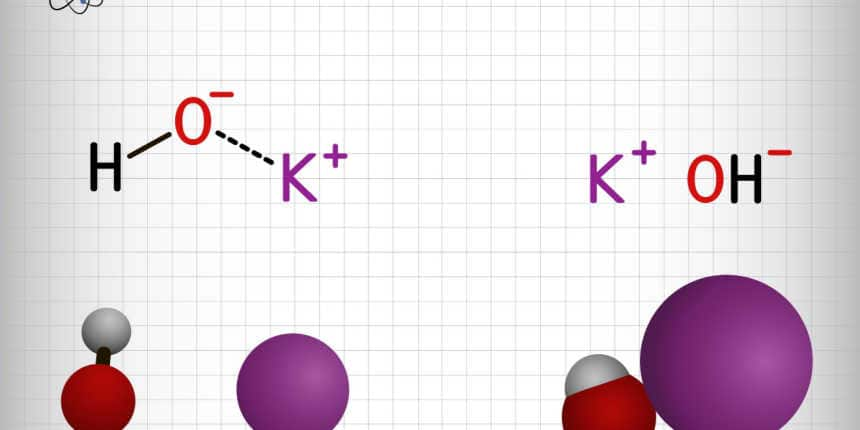
The given compound is ionic in nature, i.e., it is constituted of ions having opposite charges which attract each other by electrostatic forces. The bond length, i.e., the distance between the centres of the two ions is about 2.69 to 2.15 Å.

Production
Conventionally, Potassium hydroxide KOH was made by adding Potassium carbonate to a strong solution of Calcium hydroxide Ca(OH)2. The reaction equation for the same is as follows:
K2CO3 + Ca(OH)2 ? CaCO3 + 2KOH
The solution thus produced is then boiled to precipitate the Calcium carbonate CaCO3. The above method was used until the electrolytic method of production wasn’t discovered, until the 19th century.
The now used method involves the electrolysis of a dilute solution of Potassium chloride (KCl). Electrolysis involves passing electricity through a solution of electrolyte in order to carry out a non-spontaneous reaction. Here, the non-spontaneous reaction involves the release of Hydrogen gas (H2) at cathode and the release of Chlorine gas (Cl2) at anode. The reaction equation for the same is as follows:
2KCl + 2H2O ? 2KOH + Cl2 + H2
Cation
The ion that loses electrons to obtain positive charge is known as the cation. It undergoes oxidation easily, and thus must have a highly positive oxidation potential. For Potassium, this value is about 2.93 V. This is because once Potassium loses one electron from its outermost orbital, it obtains a noble gas configuration- which are the least reactive and most stable. This can further be understood from the electronic configurations of Potassium and noble gas Argon as given below
Potassium (K): 1s22s22p63s1
Potassium ion (K+): 1s22s22p6
Argon (Ar): 1s22s22p6
Anion
The ion that gains electrons to obtain negative charge is known as the anion. It undergoes reduction easily, and thus must have a highly positive reduction potential. For the reaction O2(g) + 2H2O(aq) + 4e- ? 4OH-(l), this value is about 0.401 V. As the electrolyte KOH ionises in water, the OH- ions are responsible for the basicity of the solution. As the dissociation of ions becomes easier due to larger size down the group, KOH is highly basic. It is among the strongest bases of inorganic chemistry as it dissociates completely.
Lattice Structure
The solid KOH crystallises into a NaCl crystalline structure at high temperatures, i.e., rock salt structure. Either randomly or rapidly, the OH- group is disordered. As a result, the OH- group is functionally a spherical anion with a radius of 1.53 Å and a size that falls between Cl and F.
At room temperature, however, it distorts the environment around K+ centres and orders the OH- group. The K+ OH- distances range from 2.69 to 3.15 Å, depending on how the OH- group is oriented.
Physical Properties
Physical characters are those aspects of the matter that are unaffected by changes in its chemical composition. Examples include solubility, boiling point, and others.
Molecular weight: It is the average mass of a compound's molecules, measured as the sum of the atomic weights of its constituent atoms and equivalent to 12 the mass of carbon 12. For Potassium hydroxide, this value is 56.11 g/mol.
Density: It refers to how compact a substance is. It is the substance's mass per unit of volume in mathematical terms. For KOH, the value is 2.12 g/cm3.
Boiling point: The temperature at which a liquid's vapour pressure equals the pressure around it and the liquid transforms into a vapour is known as the boiling point of a substance. The boiling point of KOH is 1327oC.
Melting point: The temperature at which a substance transforms from a solid to a liquid is known as its melting point. The solid and liquid phases are in equilibrium at the melting point. The melting point of KOH is 360oC.
Solubility: The ability of a material, the solute, to combine with another substance, the solvent, is known as solubility. The solubility of KOH in water at 0oC is 85 g/ 100 mL and at 25oC it is 121 g/ 100 mL.
Chemical Properties
Ionic nature: Potassium hydroxide or KOH, has an ionic nature. It is formed due to the electrostatic attraction between the cation K+ and the anion OH-.
pH: An aqueous solution's acidity or basicity can be determined using the pH scale. The pH values of acidic solutions are typically lower than those of basic or alkaline solutions. At room temperature, the pH of 1 Molar KOH solution is about 14.
Enthalpy of fusion: Also known as the heat of fusion, it is the shift in enthalpy that occurs when a particular quantity of a substance receives energy, usually heat, changing its state from a solid to a liquid at constant pressure.
Autoignition temperature: The lowest temperature at which a substance will spontaneously ignite in a normal atmosphere without the aid of an external source of ignition, such as a flame or spark, is known as the autoignition temperature or kindling point of that substance. However, KOH is not a flammable substance.
Decomposition: It is the action or result of breaking down a single chemical compound into two or more pieces. It releases hazardous K2O vapours when heated to the point of decomposition.
Uses
Used to make other potassium compounds: Potassium hydroxide is a strong base. It reacts with different acids in order to produce potassium salts like potassium carbonate (K2CO3), potassium chloride (KCl), potassium sulphate (K2SO4), etc.
Used to manufacture soft soaps: Potassium based soaps are softer than those made with sodium hydroxide. Soft soaps are made by the saponification of fats with Potassium hydroxide. Soaps made of potassium are softer and more soluble. Consequently, they liquefy more quickly and can hold more cleaning agent than liquefied sodium soaps.
Used in batteries: In nickel-cadmium, nickel-hydrogen, and manganese dioxide-zinc alkaline batteries, aqueous potassium hydroxide is used as the electrolyte. This is because the solutions of potassium hydroxide are more conductive than those of sodium hydroxide. Potassium has a greater ionic radius and thus a smaller hydration radius. This allows it to have greater mobility and thus higher conductivity.
Used in the food industry: Potassium hydroxide serves as a food thickening, pH regulator, and food stabiliser in food products.
Used for corrosive property: Potassium hydroxide's corrosive characteristics make it a valuable component of cleaners and disinfectants for materials and surfaces that can withstand their own corrosion by KOH.
Used in chemical cremation: In both animals and humans, potassium hydroxide speeds up the breakdown of soft tissues, sparing only the bones and other hard structures. Entomologists can use a 10% aqueous solution of KOH to carry out this procedure in order to investigate the intricate details of insect anatomy.
Frequently Asked Questions (FAQs)
The pH will be 14.
KOH feels soapy on touching. This is because on coming in contact with the skin, it instantly reacts with the fats present on the skin. Therefore, the fats present on the skin convert rapidly to give soap and glycerol as a product. The reaction followed is as follows:
KOH + RCOOR' → RCOOK + R'OH
Here, if R is a long chain, the product formed is called a potassium soap. Its unique quality is that it can produce lather even with seawater.
KOH is hygroscopic in nature. Therefore, it’d absorb water and form either a monohydrate KOH.H2O, a dihydrate KOH.2H2O or/and a tetrahydrate KOH.4H2O. It readily takes in water and carbon dioxide from the air, thus deliquesces.
Enthalpy change of solution is described as the difference in enthalpy connected to the substance that is dissolving in a solvent at constant pressure that is resulting in infinite dilution. Its value is 53.51 kJ/mol for KOH dissolved in water.
The solubility of a substance depends on the like-dissolves-like rule, for most substances. According to this rule, polar substances dissolve other polar substances whereas non-polar substances dissolve other nonpolar substances. Since potassium hydroxide is a polar substance, it is dissolvable in other polar substances like water, ethanol, methanol, etc., irrespective of whether they are organic or inorganic. In fact, much heat is produced when KOH is dissolved in water or alcohol.
