N/10 HCl Full Form
What is N/10 HCL?
An aqueous solution of hydrogen chloride is known as hydrochloric acid, commonly referred to as muriatic acid. It has an strong odour and is colourless. It is categorised as a powerful acid. In the digestive systems of the majority of animal species, including humans, it is a part of the gastric acid.
What is the meaning of N/10 HCl
1N HCl means, 1g equivalent or 36.5g of (Hydrochloric acid) dissolved in 1L solution. So N / 10 HCl solution means, when 0.1- gram equivalents of a given compound are added to form 1L of a solution.An aqueous solution of hydrogen chloride is what is known as hydrochloric acid, commonly referred to as muriatic acid. It has an unmistakably strong odour and is colourless. It is categorised as a powerful acid. In the digestive systems of the majority of animal species, including humans, it is a part of the gastric acid. A crucial industrial chemical and reagent for laboratories is hydrochloric acid.
The normalcy of the solution is the amount of gram equivalent mass of solute dissolved in one litre of the solution.
The mass of an element (compound or ion) that combines or displaces 1.008 g of hydrogen, 8 g of oxygen, or 35.5 g of chlorine is known as gram equivalent mass.
Consequently, the mathematical relationship is as follows:
Normality= ${\frac{number of gram equivalents mass}{volume of solution in litres}}$
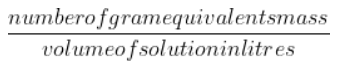
Gram Equivalents= ${\frac{{weight of solute}}{{[Equivalent weight of solute]}}}$
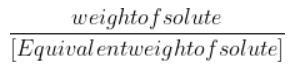
Explanation:
One mole of HCl weighs 1.008 + 35.453 = 36.461 g, or 1 gram equivalent of (hydrochloric acid) dissolved in a 1 litre solution will form 1N HCl solution.
According to the definition of normalcy, N/10 HCl solution is in which 0.1 gram equivalent of HCl has been dissolved in 1L solution.
N/10 solution, in general means when 0.1 gram equivalent of a compound are dissolved in 1L of solution.
Preparation of 1000 ml of N/10 HCl acid solution
- We know that 36 g of HCl in 1000 ml = 1 N.
- Then, 1180 g of HCl in 1000 ml will be = 1 x 1180 N /36 = 32.78 N.
- But the assay (purity) of the commercially available HCl solution = 36%
- Therefore, the actual normality will be =(36/100) x 32.78 = 11.79 N.
Molarity of HCl
Molarity (M) is the most commonly used term to describe the concentration of a solution. It is equal to the moles of solute divided per 1 litre of solution.
- M/10 HCl = 0.1 M HCl solution = 0.1 moles / L
- No. Of moles = ${\frac{Molar mass of compound}{molar mass}}$
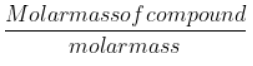
•:- 0.1= ${\frac{x}{36.5}}$
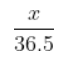
•X = ${{36.5} \times {0.1}}$ ![]() = 3.65g of HCl (dissolved in 1 L or 1000 ml of solution)
= 3.65g of HCl (dissolved in 1 L or 1000 ml of solution)
More about HCl and it’s uses
When hydrogen chloride gas and water are combined, hydrochloric acid is produced. Muriatic acid is the name for this aqueous solution of hydrochloric acid. It is an odourless gas with an unpleasant taste. A hydrogen to chlorine ratio of one to one makes up each molecule of HCl. It is a chemical compound that is both highly corrosive and potentially poisonous.
Muriatic acid, also known as hydrochloric acid, is a toxic, corrosive hazardous liquid that reacts with the majority of metals to produce explosive hydrogen gas. It also irritates the eyes and mucus membranes and causes severe burns. It is created by absorbing water containing hydrogen chloride. Acid is primarily a byproduct of chlorination. Combining hydrogen and chlorine produces pure acid.
Uses for hydrochloric acid
The fact that our stomachs naturally produce hydrochloric acid, which aids in food digestion, is one noteworthy aspect of the acid. The stomach contents become acidic due to hydrochloric acid so that enzyme pepsin can split the protiens.
Several industries, including rubber, textiles, and even photography, use this acid extensively. It is also a typical household item. Industrially, it is used to treat steel, make batteries, make fireworks, and other things. Here are some of the most typical applications for hydrochloric acid:
Common applications for hydrochloric acid
- Creation of Organic Substances
- Making Inorganic Compounds
- Getting rid of metal stains
- Production of oil
- Production of Organic Compounds, Table Salt Purification, and pH Control
- Vinyl chloride and other organic compounds are produced using hydrochloric acid.
Frequently Asked Questions (FAQs)
N/10 HCl solution is defined as when 0.1 - gram equivalents or 3.65g of HCl are added to water to form 1L of solution.
The amount of gram or mole equivalents of solute present in a solution that contains in one litre of solution is what is referred to as normality according to the accepted definition.
Formula for Normality:-
- Normality = ${\frac{[volume of solution in litres]}{[number of gramme equivalents]}}$
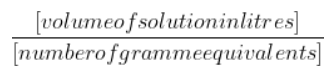
- Weight of the solute divided by the number of grammes equals the solute's equivalent weight is -1.
- 1 N = ${\frac{[Equivalent weight Volume (L)]}{Weight of Solute (gramme)}}$
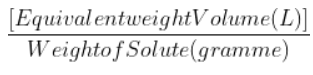
Formula of Molarity:-
M= No. of moles/Volume of solution(in L)
M=molar concentration
n=moles of solute
V=litres of solution
The formula of Normality:-
N = ${\frac{Eq}{v}}$
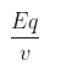
N= Normality
Eq = number of gram equivalent of solute
V = volume of solvent in litres
