Na2CO3 Full Form
What is the full form of Na2CO3?
The chemical compound sodium carbonate is inorganic. The substance typically referred to as soda ash is sodium carbonate. To produce soda ash, trona is used. Trona, a double salt made up of sodium carbonate and sodium hydrogen carbonate, is a product of the several evaporation processes in lakes. Sodium carbonate is the most crucial of all the heavy basic compounds. Since it is non-corrosive and consequently safer to handle than sodium hydroxide, this is a significant advantage. The Solvay process, the Labnac process, the dual process, and the electrolytic process are the methods for the production of sodium carbonate, as it is a weak acid. It is only marginally soluble in ethanol and insoluble in alcohol. The use of Na_{2}CO_{3} ![]() as a water softener is among its most widespread uses. The pH is somewhere about 11.
as a water softener is among its most widespread uses. The pH is somewhere about 11.
Structure of Sodium Carbonate
Sodium carbonate, with the chemical formula Na_{2}CO_{3}![]() is a diazonium salt of carbonic acid. This inorganic substance is water soluble, and when it dissolves in water, it produces sodium hydroxide and carbonic acid. It is an odourless, white powder when in its purest form.
is a diazonium salt of carbonic acid. This inorganic substance is water soluble, and when it dissolves in water, it produces sodium hydroxide and carbonic acid. It is an odourless, white powder when in its purest form.
Synthesis
The Solvay method is now the only one used to produce sodium carbonate. A cold, saturated sodium chloride solution is treated in this method with ammonia and carbon dioxide. The procedures involve almost total precipitation of sodium hydrogen carbonate, which is relatively slightly soluble in the presence of sodium ions. Filtration is used to remove it, and the resulting sodium carbonate is burned.
This procedure requires simple, affordable ingredients that are widely available. The primary ingredients for the process are calcium carbonate \left ( CaCO_{3} \right ) ![]() , ammonia \left ( NH_{3} \right )
, ammonia \left ( NH_{3} \right )![]() , and salt brine \left ( NaCl \right )
, and salt brine \left ( NaCl \right ) ![]() . \left ( CaCl_{2} \right )
. \left ( CaCl_{2} \right ) ![]() is a crucial byproduct produced in this process.
is a crucial byproduct produced in this process.
The subsequent equation can be used to describe the reactions. In order to produce it, the hydrates are heated. Additionally, it can be produced by the Solvay process, which involves heating (calcining) sodium hydrogen carbonate:
2NH_{3}+H_{2}O+CO_{2}\right arrow \left ( NH_{4} \right )_{2}CO_{3}
![]()
\left ( NH_{4} \right )_{2}CO_{3}+H_{2}O+CO_{2}\rightarrow 2NH_{4}HCO_{3}
![]()
When adding common salt to a solution of NH_{4}^{+} ![]() and HCO_{3}^{-}
and HCO_{3}^{-} ![]() , the least soluble form of HCO_{3}
, the least soluble form of HCO_{3} ![]() , NaHCO_{3}
, NaHCO_{3} ![]() , precipitates. Once removed, it is filtered.
, precipitates. Once removed, it is filtered.
NH_{4}HCO_{3}+NaCl\rightarrow NH_{4}Cl+NaHCO_{3}
![]()
Na_{2}CO_{3} ![]() is produced by heating sodium bicarbonate.
is produced by heating sodium bicarbonate.
2NaHCO_{3}\rightarrow Na_{2}CO_{3}+CO_{2}+H_{2}O![]()
The CO_{2} ![]() gas that was evolved can be recycled.
gas that was evolved can be recycled.
When dissolved in water, anhydrous sodium carbonate re-crystallizes into crystals of washing soda, each containing 10 water molecules.
Physical Properties of Sodium Carbonate (Na2CO3)
Sodium Carbonate chemical formula = Na_{2}CO_{3}
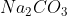
Density = 2.54 g/cm3
Boiling Point = 1600oC
Melting Point = 851oC
It’s Molecular mass = 105.9888 g/mol
White crystallized solid in colour.
Extremely soluble in water and has no flavour or smell.
Chemical Properties of Sodium Carbonate (Na2CO3)
The sodium carbonate anhydrous is heat stable. At 852oC, it melts without decomposing.
Due to the release of OH^{-}\left ( aq \right )
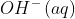 ions during hydrolysis, sodium carbonate aqueous solutions have a mildly alkaline pH.
ions during hydrolysis, sodium carbonate aqueous solutions have a mildly alkaline pH.
Na_{2}CO_{2}\left ( s \right )+2H_{2}O\left ( l \right )\rightarrow H_{2}CO_{3}\left ( aq \right )+2Na^{+}\left ( aq \right )+2OH^{^{-}}\left ( aq \right )
![]()
When weak vegetable acids, such as lime juice, combine with sodium carbonate, carbon dioxide is released.
Na_{2}CO_{3}\left ( aq \right )+2H^{+}\left ( aq \right )\rightarrow 2Na^{+}\left ( aq \right )+H_{2}O\left ( l \right )+CO_{2}\left ( g \right )
![]()
Na_{2}CO_{3}\left ( aq \right )+2HCl\left ( aq \right )\rightarrow 2NaCl\left ( aq \right )+H_{2}O\left ( l \right )+CO_{2}\left ( g \right )
![]()
When aqueous sodium carbonate solutions absorb carbon dioxide from the air, sodium hydrogen carbonate is produced.
Na_{2}CO_{3}\left ( aq \right )+H_{2}O+CO_{2}\left ( g \right )\rightarrow 2NaHCO_{3}\left ( aq \right )
![]()
Uses of Sodium Carbonate(Na2CO3)
It functions as an abrasive and foaming agent in toothpaste.
It is used to make borax, sodium phosphate, water glass (sodium silicate), and a number of other salt compounds.
It serves as a pH adjuster.
It is employed in the production of paper, soap, and detergents.
In the brick industry, it functions as a wetting agent.
It is used as a water softener because carbonate precipitates hard water's magnesium and calcium ions.
Frequently Asked Questions (FAQs)
In Wyoming, it is found in large natural quantities, and it is mined there. In California, it is also extracted from lake brines (with other chemicals). Glass manufacturing and chemical manufacturing are two of sodium carbonate's principal applications.
A weak acid and a strong base combine to form salt, which is basic in nature. Sodium carbonate is made by combining carbonic acid and sodium hydroxide.
Numerous health and safety risks could be posed by sodium carbonate. This chemical inhalation may have unfavourable effects such as pulmonary edema, coughing, respiratory tract irritation, and shortness of breath.
Sodium carbonate decahydrate (natron), Na_{2}CO_{3}.10H_{2}O ![]() , Sodium carbonate heptahydrate, Na_{2}CO_{3}.7H_{2}O
, Sodium carbonate heptahydrate, Na_{2}CO_{3}.7H_{2}O ![]() , and Monohydrate of sodium carbonate, Na_{2}CO_{3}
, and Monohydrate of sodium carbonate, Na_{2}CO_{3} ![]() , in water. It's also known as crystal carbonate.
, in water. It's also known as crystal carbonate.
With the chemical formula Na_{2}CO_{3}.nH_{2}O ![]() hydrated sodium carbonate completely breaks down into water and anhydrous sodium carbonate when heated vigorously.
hydrated sodium carbonate completely breaks down into water and anhydrous sodium carbonate when heated vigorously.