O3 Full Form
What is the full form of O3?
In chemistry, the full form of O3 is ozone. Three oxygen atoms make up the chemical known as ozone, or O3. Ozone tends to start out as invisible pollution, which may be difficult to comprehend. We frequently refer to ozone by its older, more familiar term, “smog,” when it accumulates and mixes with other contaminants. O3 is the chemical formula for ozone, a kind of oxygen. Ozone, a substance that exists naturally in the stratosphere of the planet, contributes to the sun's UV radiation absorption. Ozone has both positive and negative effects on life on Earth, depending on its location in the atmosphere. The ozone layer is damaged by the hazardous compounds that are released by the chlorofluorocarbons in aerosols and other products used in refrigeration. Humans are consequently exposed to UV radiation, which can lead to a variety of skin issues.
- What is the full form of O3?
- Chemical structure of Ozone (O3)
- Ozone Layer
- Benefits of Ozone

What is Ozone?
The chemical formula for ozone is O3. Three oxygen atoms make up the highly reactive gas known as ozone (O3). It occurs in the stratosphere, the upper atmosphere of the earth, and the lower atmosphere of the planet (the troposphere). Ozone, a substance that exists naturally in the stratosphere of the planet, contributes to the sun's UV radiation absorption.
How is Ozone (O3) formed?
O3 is formed when UV radiation interacts with oxygen or dioxygen molecules. The oxygen molecule is broken up into free oxygen atoms by the UV rays. Ozone is formed when these O atoms join with oxygen in its molecular state. It is a thermodynamically unstable substance that frequently breaks down into molecular oxygen. As a result, there is a stable dynamic equilibrium between the formation and destruction of O3 molecules.
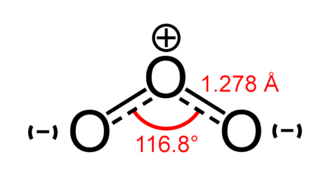
Chemical structure of Ozone (O3)
Ozone's structure consists of three oxygen atoms, but steric hindrance prevents it from creating a triangle structure with each O atom forming the expected two bonds. Instead, just one link is formed by each oxygen, and the remaining charge is dispersed throughout the molecule.
Ozone Layer
Between 15 and 30 kilometres above the surface of the earth, the ozone layer protects us and other living things from the sun's harmful UV radiation. Damage to the ozone layer is more akin to a very thin patch than a hole. One layer of the stratosphere, and the second layer of the Earth's atmosphere, is the ozone layer. The layer of insulating gases that clings to our planet is known as the stratosphere.
Ozone Layer Depletion
The stratosphere of the earth's atmosphere contains specific compounds that contribute to the thinning of the protective O3 layer. The main cause of the depletion is the continuous emission of substances like carbon tetrachloride, carbon tetrafluoride, CFCs (chlorofluorocarbons), freons, and other halogens containing chlorine or bromine into the atmosphere.
CFC compounds are organic molecules that are not reactive, poisonous, or combustible. As a result, it is utilised in the creation of plastic foam, air conditioners, refrigerators, the cleaning of computer components, etc. But before they reach the stratosphere, these compounds combine with common atmospheric gases. Therefore, when strong UV radiation is present in the stratosphere, these molecules decompose into free chlorine radicals.
Effects of Ozone Layer Depletion
The ozone layer's depletion has negative effects on the environment. Let's look at the main impacts of ozone layer depletion on people and the environment:
UV rays are extremely dangerous and can result in conditions like skin cancer, ageing of the skin, cataracts, sunburn, etc.
Fish productivity is reduced, and phytoplankton is killed as a result of O3 layer depletion. Altering the plant protein can lead to mutations in plant cells.
Animals that are directly exposed to UV light develop skin and eye cancer.
Strong UV radiation may prevent plants from growing, blooming, or performing photosynthesis. The detrimental effects of UV light must also be endured by the woodlands.
Benefits of Ozone
- Without filtration systems, ozone is employed in water treatment plants.
- Photocopiers, laser printers, and other electrical devices that are often used can also produce ozone.
- In medicine, ozone therapy is used to disinfect areas and treat illnesses by reducing the effects of bacteria, viruses, fungi, yeast, and protozoa.
- A number of ozone-depleting substances have qualities that make them effective refrigerants, or have the ability to effectively transport heat from one place to another.
- O3 is constantly produced on-site and is also very efficient at killing germs, viruses, and protozoa.
- Iron and manganese are two examples of pollutants that are oxidised when water disinfects.
Frequently Asked Questions (FAQs)
Ozone, whether in its purest form or when coupled with other substances, can be dangerous. In areas with high ozone pollution levels, everyone who spends time outside could be in danger. Inhaling ozone can harm the lungs. Ozone concentrations that are relatively low might lead to lung irritation, coughing, throat pain, and shortness of breath.
The easiest method to protect your health is to learn when local ozone levels are expected to be at their highest and take basic precautions. You can take the following measures in order to protect yourself from ozone:
Observing the air quality forecast, especially during hot weather.
Reducing outside activities until the air is better.
Minimising the usage of machinery powered by gasoline when possible.
The oxygen-containing double bond in ozone has two lone pairs, one lone pair for the oxygen-containing positive charge, and three lone pairs for the oxygen-containing negative charge. Thus, there are a total of six lone pairs in a single ozone molecule.
According to the VSEPR (valence shell electron pair repulsion) theory, the electron cloud surrounding the two oxygen atoms on either end will be repelled by electrons. This will cause the end O groups to be pulled downward, giving the O3 molecule a bent or V-shaped molecular geometry.
The Earth is shielded by ozone from the sun's harmful ultraviolet (UV) rays. Life on Earth would be very challenging without the ozone layer in the atmosphere. Plants and planktons, which provide nutrition to the majority of ocean life, cannot survive or grow in the presence of extreme ultraviolet radiation. With a thinner Ozone Layer shield, humans would be more susceptible to skin cancer, cataracts, and immune system impairment.

