pH Full Form
What is the full form of PH ?
The full form of Ph is the potential of hydrogen, the pH scale is used to define the acidity or basicity of an aqueous solution or to measure the hydrogen ion activity of solutions. Historically, pH stands for "potential of hydrogen" (or "power of hydrogen"). Lower pH values are recorded for acidic solutions (solutions with greater H+ ion concentrations) than for basic or alkaline solutions. The pH is logarithmic (to the base 10) and inversely proportional to the hydrogen ion concentration in the solution or pH is the negative logarithm (to the base 10) of hydrogen ion activity as depicted in the equation below.
pH=-log_{10}\left [ H ^{+}\right ], ![]()
Acidic solutions have a pH below 7, while basic solutions have a pH above 7, at 25 °C (77 °F). At this temperature, solutions with a pH of 7 are neutral (i.e., having an equal amount of H+ and OH- ions, such as pure water). If the temperature rises over 25 °C, the pH neutral value falls below 7, and vice versa. For extremely concentrated strong acids, the pH value can be less than 0; for very concentrated strong bases, it can be larger than 14.
History of pH
The notion of pH was initially proposed in 1909 at the Carlsberg Laboratory by the Danish scientist Søren Peder Lauritz Sørensen. It was later updated to the contemporary pH in 1924 to accept definitions and measurements in terms of electrochemical cells. The initial studies used the notation pH•, which included the subscript H• after the lowercase p. Due to Sørensen's failure to provide context for his usage of the letter p in "pH," its exact meaning is uncertain. Sørensen outlines a method for calculating pH using potential differences, where pH is the negative power of 10 of the concentration of hydrogen ions. The letter p can be used to denote "power" or "potential," as in the words puissance (French), potenz (German), and potens (Danish). Some literary sources that claim "pH" stands for the Latin words pondus hydrogenii (amount of hydrogen) or potentia hydrogenii (power of hydrogen), although Sørensen's works do not support this. The letter p is now used in chemistry to denote "decimal logarithm of," as well as in the terms pKa for acid dissociation constants and pOH for hydroxide ions.
Methods to determine the pH
A device known as a conductivity meter is used to determine the amount of electrical current in a solution. By definition, bases release hydroxide ions, while acids release hydrogen ions in solution. Both of these species are electrically charged, and it is the responsibility of the conductivity meter is to determine both the charge's presence and the relative concentration of ions. The device can determine the pH of the solution by knowing the concentration.
A pH meter is another device chemists used to determine a solution's pH. A conductivity meter and a pH meter have many similarities. However, the hydrogen ion concentration inside a solution is what the pH meter specifically looks for and detects. In order to assure accuracy, a pH meter must normally be calibrated. This is typically done by using a reference solution whose precise pH is known. Unknown pH readings are first calculated in relation to the standard after the meter is calibrated to that particular value.
If we needed to know about a solution's pH is either acidic or basic, rather than its precise numerical value, a pH indicator can be used. A pH indicator is a substance that alters the colour of the solution depending on the environment of the solution in which it is present. For instance, the chemical molecule methyl orange has red colour in acidic medium while it shows yellow colour in alkaline medium.
Nernst equation plays a very important role in determining the pH of a solution when using a conductivity cell or a pH meter.
E=E^{\circ }+\frac{RT}{F}ln\left ( a_{H^{+}} \right )=E^{\circ }-\frac{2.303RT}{F}pH
![]()
As we know,pH=-log_{10}\left [ H ^{+}\right ] and ln=2.303log
![]() and
and ![]()
Where,
E^{\circ } , ![]() = standard electrode potential
= standard electrode potential
E = measured electrode potential
R = gas constant
T = temperature in Kelvin
F = Faraday constant,
Therefore if pH is described in terms of activity, the electrode potential must be proportional to pH.
pH of Scale
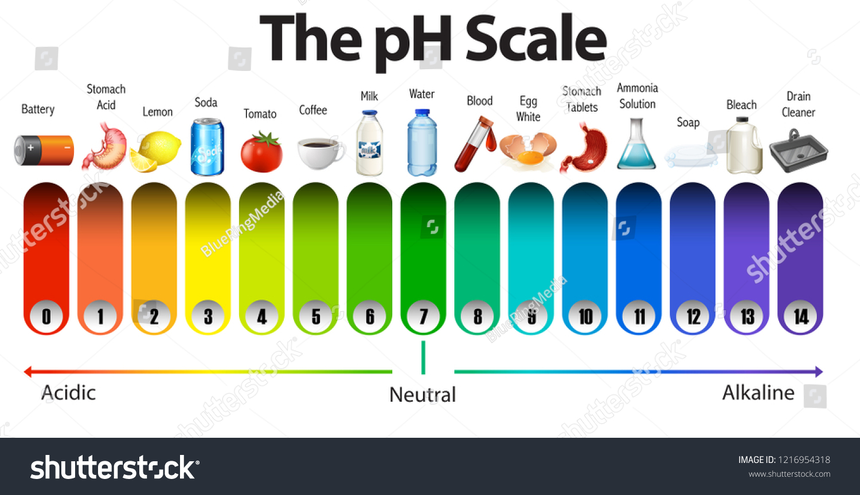
To determine the extent of basicity or acidity of a substance, the pH scale is widely used. The pH scale has values ranging from 0 to 14. The pH scale has a range of 1 to 7 for acidic substances, with 1 being the most acidic, and a range of 7 to 14 for alkaline or basic compounds with 14 being the most basic substance. The pH of a substance that is completely neutral would be 7.
The pH scale may be traced to a group of standard solutions whose pH has been decided upon by international consensus. By detecting the potential difference between a hydrogen electrode and a standard electrode, such as the silver chloride electrode, the primary pH standard values are calculated using a concentration cell with transference. A glass electrode and pH meter, as well as a colour-changing indicator, can be used to determine the pH of aqueous solutions.
pH=-log_{10}\left [ H^{+} \right ]
![]()
pOH=-log_{10}\left [ OH ^{-}\right]
![]()
pH+pOH=14
![]()
Applications of pH
In the field, the soil is a heterogeneous colloidal system made up of sand, silt, clays, microbes, plant roots, and numerous other living species and decomposing organic matter. For soil and environmental scientists, farmers, and engineers, soil pH is a key variable that impacts a wide range of processes and qualities.
Numerous plants have pH-dependent pigments that can serve as pH indicators, such as hibiscus, red cabbage (anthocyanin), and grapes (red wine). Citric acid is the primary component that makes citrus fruit juice acidic.
Seawater's pH normally only fluctuates between 7.4 and 8.5. It is crucial to the ocean's carbon cycle and there is evidence that carbon dioxide emissions are contributing to continuous ocean acidification. The chemical makeup of seawater complicates pH measurement, and there are a number of different pH scales used in chemical oceanography.
In a process known as acid-base homeostasis, the pH of various cellular compartments, bodily fluids, and organs is typically strictly maintained. Acidosis, or an excess of acid in the body, is the most prevalent condition of acid-base balance and is often indicated by a pH level below 7.35. The opposite disorder, alkalosis, is marked by abnormally high blood pH. Outside of their optimal pH range, enzymes and other proteins in the body might lose their functionality or become denatured.
Frequently Asked Questions (FAQs)
The pH of pure water is 7 (neutral), it is neither acidic nor basic while the recommended pH of drinking water can range between 6.5 to 8.5.
Human blood is slightly basic in nature and its value ranges between 7.35 to 7.45 while the stomach fluids of humans are highly acidic in nature and their value ranges between 1.5 to 3.5.
The acidity or alkalinity of solutions is indicated by a universal indicator, which is a pH indicator made of a solution of many compounds that shows multiple smooth colour changes across a wide range of pH values.
A buffer is a substance that can withstand a pH shift when acidic or basic components are added. Small additions of acid or base can be neutralised by it, helping to keep the pH of the solution comparatively stable.
Milk is considered to be slightly acidic due to the presence of lactic acid in milk.