Binding Energy Per Nucleon
Binding energy per nucleon refers to the average energy that holds a nucleus together, calculated by dividing the total binding energy of the nucleus by the number of nucleons (protons and neutrons) it contains. This concept is crucial in nuclear physics as it explains the stability of different nuclei; higher binding energy per nucleon typically indicates greater stability. In real life, understanding binding energy per nucleon is essential for applications such as nuclear power generation, where energy is released by fission or fusion reactions, and in medical treatments like cancer radiotherapy. This article explores the significance of binding energy per nucleon and its practical implications in both scientific and everyday contexts, related terms to it and solved examples for better understanding.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
Energy Mass Equivalence
Energy-mass equivalence, articulated by Albert Einstein in his famous equation E=mc2, states that energy and mass are interchangeable; a small amount of mass can be converted into a large amount of energy and vice versa. This principle underlies many fundamental processes in physics, such as nuclear reactions, where the conversion of a tiny fraction of mass results in immense energy release.
Einstein showed from his theory of special relativity that it is necessary to treat mass as another form of energy. Einstein showed that mass is another form of energy and one can convert mass into other forms of energy, say kinetic energy and vice-versa. For this Einstein gave the famous mass-energy equivalence relation
E=mc2 where c is the velocity of light in vacuum and c=3×108 m/s
or we can say ΔE=Δmc2
where Δm= mass defect and ΔE= energy released
Note:
- For a mass defect equal to 1 amu, the energy released is ΔE=(Δm)c2=(1amu)×(3×108)2=931.5MeV
- A small amount of mass corresponds to a large amount of energy. Energy associated with the rest mass of an object is said to be its rest mass energy.
| Rest mass of an electron (me) | 9.1 x 10-31 kg |
| 5.485 x 10-4 amu | |
| Rest mass of a proton (mp) | 1.6726 x 10-27 kg |
| 1.00727 amu | |
| 1836.15 me | |
| Rest mass of a neutron (mn) | 1.6749 x 10-27 kg |
| 1.0086 amu | |
| Energy equivalence of rest mass of an electron | 0.51 MeV |
| Energy equivalence of rest mass of a proton | 938.27 MeV |
| Energy equivalence of rest mass of a neutron | 939.56 MeV |
It is very useful to calculate energy emitted in the nuclear process.
What is a Mass Defect?
Mass defect refers to the difference between the mass of an atomic nucleus and the sum of the masses of its protons and neutrons. This phenomenon occurs because a portion of the mass is converted into binding energy, which holds the nucleus together.
It is found that the mass of a nucleus is always less than the sum of the masses of its constituent nucleons in a free state.
This difference in masses is called the mass defect.
Hence mass defect is given as
Δm= Sum of masses of nucleons- Mass of the nucleus Δm=[Zmp+(A−Z)mn]−M
where
mp= Mass of proton, mn= Mass of each neutron,
M= Mass of nucleus, Z= Atomic number, A= Mass number,
Note- The mass of a typical nucleus is about 1% less than the sum of masses of nucleons.
What is a Packing Fraction?
Packing fraction is a concept in nuclear physics that quantifies the average binding energy per nucleon in a nucleus. It is defined as the mass defect per nucleon, expressed as a fraction of the atomic mass unit (AMU).
Mass defect per atomic mass number is called packing fraction. The packing fraction measures the stability of a nucleus. The smaller the value of the packing fraction, the larger the stability of the nucleus.
Packing fraction (f)=ΔmA=M−(Zmp+(A−Z)mn)A
mp= Mass of proton, mn= Mass of each neutron,
M= Mass of nucleus, Z= Atomic number, A= Mass number
Packing Fraction can have positive, negative, or zero values.
The zero value of the packing fraction is found in monoisotopic elements where the isotopic mass is equal to the mass number. For For 8O16f→ zero
A negative value of packing fraction indicates that there is a mass defect, hence binding energy. Such nuclei are stable.
Positive values of the Packing fraction are unstable when undergoing fission and fusion processes.
Nuclear binding energy (B.E)
Nuclear binding energy (B.E) is the energy required to disassemble a nucleus into its constituent protons and neutrons. It represents the energy released when a nucleus is formed from these nucleons and is a measure of the stability of the nucleus. The greater the binding energy, the more stable the nucleus. The neutrons and protons in a stable nucleus are held together by nuclear forces and energy is needed to pull them infinitely apart. This energy is called the binding energy of the nucleus.
OR
The amount of energy released when nucleons come together to form a nucleus is called the binding energy of the nucleus.
OR
The binding energy of a nucleus may be defined as the energy equivalent to the mass defect of the nucleus.
If Δm is a mass defect then according to Einstein's mass-energy relation
then
Binding energy =Δm×c2=[{mpZ+mn(A−Z)}−M]×c2J Binding energy =Δm×931.5MeV
To see how nucleon binding energy varies from nucleus to nucleus, it is important to compare the binding energy on a nucleon basis. A more useful measure of the binding between protons and neutrons is the binding energy per nucleon or Ebn. It is the ratio of the binding energy of a nucleus to the number of nucleons in the nucleus:
Ebn=EbA or Ebn=ΔMc2 A
where, A = Number of Nucleons.
We can define binding energy per nucleon theoretically as the average energy per nucleon needed to separate a nucleus into its individual nucleons.
Let’s look at a plot of the binding energy per nucleon versus the mass number for a large number of nuclei:

The following main features of the plot are
- The maximum binding energy per nucleon is around 8.75 MeV for mass number (A) = 56.
- The minimum binding energy per nucleon is around 7.6 MeV for mass number (A) = 238.
- The binding energy per nucleon, Ebn, is practically constant, i.e. practically independent of the atomic number for nuclei of middle mass number ( 30 < A < 170).
- Ebn is lower for both light nuclei (A<30) and heavy nuclei (A>170).
We can draw some conclusions from these four observations:
Conclusion 1
The force is attractive and sufficiently strong to produce a binding energy of a few MeV per nucleon.
Conclusion 2
- Ebn is nearly constant in the range 30 < A < 170 because the nuclear force is short-ranged. Consider a particular nucleon inside a sufficiently large nucleus. It will be under the influence of only some of its neighbours, which come within the range of the nuclear force.
- This means that all nucleons beyond the range of the nuclear force form NA will have no influence on the binding energy of NA. So, we can conclude that if a nucleon has ‘p’ neighbours within the range of the nuclear force, then its binding energy is proportional to ‘p’.
- If we increase A by adding nucleons they will not change the binding energy of a nucleon inside. Since most of the nucleons in a large nucleus reside inside it and not on the surface, the change in binding energy per nucleon would be small.
- The binding energy per nucleon is a constant and is equal to pk, where k is a constant having the dimensions of energy. Also, the property that a given nucleon influences only nucleons close to it is also referred to as the saturation property of the nuclear force.
Conclusion 3
A very heavy nucleus, say A = 240, has lower binding energy per nucleon compared to that of a nucleus with A = 120. Thus if a nucleus A = 240 breaks into two A = 120 nuclei, nucleons get more tightly bound. Also, in the process energy is released. This concept is used in Nuclear Fission.
Conclusion 4
Now consider two very light nuclei with A < 10. If these two nuclei were to join to form a heavier nucleus, then the binding energy per nucleon of the fused and heavier nucleus would be more than the Ebn of the lighter nuclei. So, the nucleons are more tightly bound post-fusion. Again energy would be released in such a process of fusion. This is the energy source of the sun.
What is Nuclear Force?
Coulomb force is a force that determines the motion of atomic electrons. As in the previous concept, we have seen that for average mass nuclei, the binding energy per nucleon is approximately 8 MeV, This is much larger than the binding energy in atoms. Hence, the nuclear force required to bind a nucleus together must be very strong and of a different type. It must be strong enough to overcome the repulsion between the (positively charged) protons and to bind both protons and neutrons into the tiny nuclear volume.
Le's look at the features of this force also called the nuclear binding force which is obtained from many experiments which were performed in between 1930 and 1950.
- The nuclear force is much stronger than the Coulomb force acting between charges or the gravitational forces between masses. This is because the nuclear force needs to overpower the Coulomb repulsive force between the like-charged protons inside the nucleus. Hence, the nuclear force > the Coulomb force. Also, the gravitational force is much weaker than the Coulomb force.
- The nuclear force between two nucleons falls rapidly to zero as their distance is more than a few femtometres. This leads to a saturation of forces in a medium or a large-sized nucleus, which is the reason for the constancy of the binding energy per nucleon. Also, if the distance falls below 0.7fm, then this force becomes repulsive. A rough plot of the potential energy between two nucleons as a function of distance is shown below.
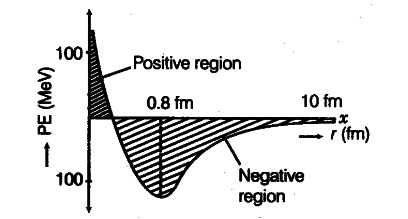
The potential energy of two nucleons is a function of the distance between them.
If distance >rα, then nuclear force = attractive
If distance <rα the nuclear force = repulsive
- The nuclear force between neutron-neutron, proton-neutron and proton-proton is approximately the same. The nuclear force does not depend on the electric charge.
Nuclear Stability
Nuclear Stability is a concept that helps to identify the stability of an isotope. The two main factors that determine nuclear stability are the neutron/proton ratio (neutron to proton ratio.) and the total number of nucleons in the nucleus.
Neutron/Proton Ratio
The graph below is a plot of the number of neutrons versus the number of protons in various stable isotopes. Stable nuclei with atomic numbers up to about 20 have an n/p ratio of about 1/1.
Above Z = 20, the number of neutrons always exceeds the number of protons in stable isotopes. The stable nuclei are located in the pink band known as the belt of stability. The belt of stability ends at lead-208.
Number of Nucleons
No nucleus higher than lead-208 is stable. That's because, although the nuclear strong force is about 100 times as strong as the electrostatic repulsions, it operates over only very short distances. When a nucleus reaches a certain size, the strong force is no longer able to hold the nucleus together.
Solved Examples Based on Binding Energy Per Nucleon
Example 1: The binding energy per nucleon of deuteron (12H) and helium nucleus (24He) is 1.1 MeV and 7 MeV respectively. If two deuteron nuclei react to form a single helium nucleus, then the energy released is :
1) 13.9 MeV
2) 26.9 MeV
3) 23.6 MeV
4) 19.2 MeV
Solution:
Q value
X+Y→Z+QQ=(Mx+My−Mz)C2
where
Mx and My are mass of reactant
Mz is the mass of the product
Energy released =E(24He)−2E(12H)=(7×4−4×1.1)MeV=23.6MeV
Hence, the answer option is (3).
Example 2: When 3Li† nuclei are bombarded by protons, and the resultant nuclei are 4Be8 the emitted particles will be :
1) neutrons
2) alpha particles
3) Beta particles
4) gamma photons
Solution:
37Li+11p→48Be+zAX
From conservation of atomic number z + 4 = 4 or z = 0
From conservation of mass number 7 + 1 = 8 + A or A = 0
So the particle is a massless particle and hence it is a gamma photon.
Hence, the answer is option (4).
Example 3: Two deuterons undergo nuclear fusion to form a Helium nucleus. The energy released (in MeV ) in this process is : (given binding energy per nucleon for deuteron=1.1 MeV and for helium=7.0 MeV)
1) 23.6
2) 32.4
3) 30.2
4) 25.8
Solution:
12D+12D→24He Energy produced =−2 m(12D)+m(24He)=(4×7)−(4×1.1)=23.6MeV
Hence, the answer is option (1).
Example 4: If the binding energy per nucleon in 37Li and 24He nuclei are 5.60 MeV and 7.06 MeV respectively, then in the reaction: p+37Li→224He the energy of the proton (in MeV ) must be :
1) 17.28
2) 28.24
3) 39.2
4) 1.46
Solution:
Q value X+Y→Z+QQ=(Mx+My−Mz)C2 wherein Mx and My are mass of reactant Mz is mass of product Binding energy of 37Li=7×5.60=39.2MeV Binding energy of 24He=4×7.06=28.24MeV∴ The energy of proton = Energy of [2(24He)−37Li]=2×28.24−39.2=17.28MeV
Example 5: Find the binding energy (in MeV ) per nucleon for 50120Sn. Mass of proton mp=1.00783U, mass of neutron mn=1.00867U and mass of tin nucleus mSn=119.902199U
(take 1U = 931 MeV)
1) 8.5
2) 9.0
3) 8.0
4) 7.5
Solution:
The number of protons in 50Sn120=50 andthenumberofneutrons =120− 50=70.
=[50×1.00783u+70×1.00867u−119.902199u]c2=(1.096201u)c2=(1.096201u)(931MeV/u)=1020.563131MeV
Binding energy per nucleon =1020.563131120=8.504MeV≈8.5MeV
Hence, the answer is (8.5).
Summary
Binding energy per nucleon is a key measure of nuclear stability, indicating the average energy holding a nucleus together. Higher binding energy per nucleon generally implies greater stability, influencing nuclear reactions such as fission and fusion. Concepts like mass defect, packing fraction, and nuclear force are crucial for understanding nuclear processes and their applications in energy production and medical treatments. Solved examples illustrate practical calculations of energy release and nuclear stability in various reactions.
Also Read
28 Nov'24 05:49 PM
28 Nov'24 10:14 AM
28 Nov'24 10:06 AM
08 Oct'24 03:32 PM
24 Sep'24 05:17 PM
29 Aug'24 09:52 AM