Dielectric Constant - Definition, Formula, FAQs
What is Dielectric Constant?
The term "dielectric" refers to a substance with insulating qualities. It is a substance capable of transmitting electric force without conduction.
Many different types of electric and polarised materials have some combination property. You will come across several terminologies linked to dielectric and its applicability in today's real situations as you read this text. You'll also learn about unitless values and numbers that characterise how electric insulators behave when they're exposed to an electric field. Let’s learn the dielectric constant meaning.
- What is Dielectric Constant?
- What is Dielectric Constant Definition?
- What are dielectric constant units?

What is Dielectric Constant Definition?
Dielectric constant, also known as relative permittivity or specific inductive capacity and it is ratio of capacitance of capacitor filled with particular material to capacitance of an identical capacitor in vacuum without dielectric material.
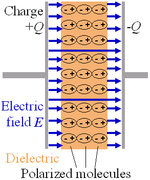
When a dielectric is introduced between the plates of a parallel-plate capacitor, the capacitance, or ability to store opposing charges on each plate, is always increased, compared to when the plates are separated by a vacuum.
The dielectric constant is represented by the Greek letter kappa, which is simply stated as $\mathrm{k}=\mathrm{C} / \mathrm{C}_0$. Here, C is the capacitance of a capacitor filled with a specific type of dielectric. And $\mathrm{C}_0$ is the capacitance of an identical capacitor in a vacuum.
The dielectric constant is a one-dimensional quantity. The dielectric constant and permittivity are the same in the centimetre-gram-second system.
Dielectric constant symbol $=\kappa$
Permittivity symbol $=\boldsymbol{\epsilon}$
Dielectric constant refers to a dielectric's large-scale feature rather than the electrical behavior at the atomic level.
Any material's static dielectric constant is always greater than one, which is its value in a vacuum.
Because the dielectric constant of air is roughly equal to that of a vacuum, air does not change the capacitance of a capacitor in any practical sense. By comparing the capacitance when the dielectric is in place to the capacitance when the capacitor is filled with air, dielectric constants of liquids and solids can be established.
Also read -
- NCERT Solutions for Class 11 Physics
- NCERT Solutions for Class 12 Physics
- NCERT Solutions for All Subjects
What are dielectric constant units?
The dielectric constant has no unit as it is the ratio of the same quantity (capacitance).
Here are some examples of dielectric constant values of various materials:
|
Dielectric Materials |
Dielectric Constant Value |
|
The dielectric constant of vacuum |
1.00 |
|
The dielectric constant of air |
1.00059 |
|
The dielectric constant of water |
80.4 at 20 degrees Celcius. |
|
The dielectric constant of germanium |
16 |
| The dielectric constant of paraffin | 2.25 |
| The dielectric constant of glass | 5-10 at 20 degrees Celcius |
NCERT Physics Notes :
Define dielectric strength.
The ability of an insulating material to act as an insulator is known as dielectric strength. The highest electric field that an insulating material can withstand before dielectric breakdown and becoming electrically conductive is defined as its dielectric strength. The highest voltage required to create a dielectric breakdown in an electrically insulating substance is also known as dielectric strength.
Point to note:
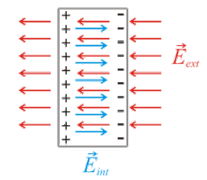
When electric charges react with an electrical conductor, they do not flow through the materials; instead, they move in position and value, resulting in dielectric polarisation. Positive charges are moved towards the field, and negative charges are displaced away from the field because the substance is polarised.
If the field shifts on the x-axis, for example, the negative charges will be directed towards the negative x-axis. If the dielectric contains weak link molecules, they will become polarised and re-oriented, allowing the symmetric axes to align.
|
Related Topics, |
Dielectric constant formula.
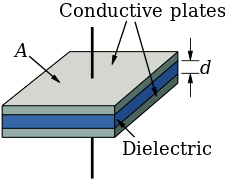
The dielectric constant is the ratio of the capacitance produced by two metallic plates in the presence of an insulator to the capacitance of the same region in the absence of an insulator.
A low dielectric constant is required when a material is utilized for any insulating purpose.
The dielectric constant formula is as follows:
$
\mathrm{k}=\mathrm{C} / \mathrm{C}_0
$
$
\mathrm{C}_0=\varepsilon_0 \mathrm{~A} / \mathrm{t}
$
$\mathrm{C}=$ capacitance of the dielectric-capacitor material
$
\mathrm{C}_0=\text { capacitance of vacuum as dielectric. }
$
T is the sample thickness.
A is the plate's surface area.
$\epsilon_0=$ Free-space permittivity $8.85 \times 10^{-12} \mathrm{~F} / \mathrm{m}$
Relation between the dielectric constant and electric susceptibility.
The dielectric constant is a measurement of how well a material conducts electricity.
When a substance is held in an electric field, its electrical susceptibility determines how polarised it becomes. If the material becomes more polarised, it will create an internal field that opposes the external field, lowering the overall electric flux through it. As a result, the medium's electric permittivity is affected by its electric susceptibility.
The lower the electric permittivity, the higher the level of polarization.
Also, check-
Frequently Asked Questions (FAQs)
A solvent's polarity is measured by its dielectric constant (symbol: ĸ). The higher a solvent's dielectric constant, the more polar it is.
The voltage necessary to create a dielectric breakdown through a material is referred to as dielectric strength. Volts per unit thickness is the unit of dielectric constant.
The dielectric constant of a medium is equal to the ratio of the substance's permittivity to the permittivity of free space.
K=ε/ε0
The dielectric constant of water is 80.4 at 20 degrees Celcius.
According to physicists, permittivity is the ability to resist external electric fields. A substance with high permittivity requires a high electric field to get polarised.
On the contrary, susceptibility is the ability to get polarised, meaning a substance with high susceptibility is easy to polarise.
Mathematically, both of these quantities are linearly dependent on each other.
Also Read
02 Jul'25 07:03 PM
02 Jul'25 06:02 PM
02 Jul'25 05:50 PM
02 Jul'25 05:34 PM
02 Jul'25 05:34 PM
02 Jul'25 04:59 PM
02 Jul'25 04:59 PM
02 Jul'25 04:49 PM
02 Jul'25 04:45 PM
02 Jul'25 04:27 PM