Electromotive Force - Definition, Formula, Unit, Difference, FAQs
When an electric current is absent, the electromotive force is the voltage at the source's terminals. The phrase "electromotive force" refers to the amount of work required to separate the charge carriers in a source current in such a way that the force acting on the charges at the source's terminals is not a direct result of the field.EMF's full form is electromotive force. In this article, we will discuss the emf definition, the SI unit of emf, the dimension of emf, the types of emf, the formula of emf, factors affecting emf, and the difference between emf and potential difference.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Electromotive Force?
- What is the Source of EMF and Define EMF of a cell?
- Formula Of EMF
- What is the SI Unit of Electromotive Force?
- Dimensional Formula Of EMF
- Difference Between EMF and Terminal Voltage
- Difference Between Electromotive Force and Potential Difference
- Factors Affecting EMF
- Types of EMF
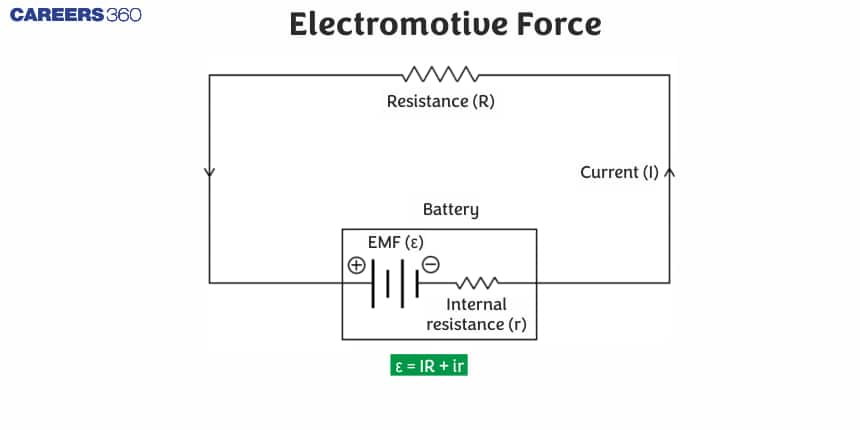
What is Electromotive Force?
Emf Definition: The amount of work done in the energy transformation (or conversion) and the electricity that travels through the electrical source or generator is defined as the electromotive force (EMF). The electromotive force (EMF) symbol is $\varepsilon$. Internal resistance leads to the development of emf.
In physics, what is EMF?
Now we will know what EMF is in physics and what EMF means in physics: When zero electric current is drawn from the cell, the electromotive force is the highest potential difference between the two electrodes. The Electromotive Force is represented by the letter E; however, it is also sometimes represented by the sign $\varepsilon$.
We know that electric charges move in an electric circuit; however, we must apply an external force to move the charges in a specific electric circuit. The electromotive force definition is that it is the force applied by the battery or an external electric source such as a battery to cause the charges to accelerate. It is not a form of force, despite its name, but rather a potential difference.
What is the Source of EMF and Define EMF of a cell?
Consider a basic circuit of a bulb connected to a battery to dispel these doubts.
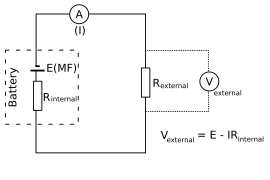
The battery (or any other electro-voltaic cell) can be thought of as a two-terminal device with one terminal at a higher potential than the other. The positive terminal, which has a higher electric potential, is sometimes referred to as the positive terminal and is usually denoted by a plus sign. The negative terminal, which is labeled with a minus sign, is the lower-potential terminal. This is referred to as the electromotive force or EMF source.
There is no flow of charges within the electromotive force source when the source is separated from the light. Charges flow from one terminal to another, passing through the bulb after the battery is reconnected to the bulb. This causes the bulb to shine. Positive charges leave the positive terminal, pass through the lamp, and enter the negative terminal of the emf source in positive current flow, also known as conventional current flow. This is how you set up an EMF source.
Now, what is the emf of a cell?
The potential difference created at both ends of a battery is the electromotive force of a cell.
|
Related Topics, |
Formula Of EMF
There are three formulas for EMF depending on the EMF generated
Induced EMF
$$
\mathcal{E}=-N \frac{d \Phi}{d t}
$$
where,
- $\mathcal{E}$ is the induced EMF
- $N$ is the number of turns in the coil
- $\Phi$ is the magnetic flux
Motional EMF
The formula for motional emf is given as:
$$
\mathcal{E}=B \cdot l \cdot v
$$
where,
- $B$ is the magnetic field strength
- $l$ is the length of the conductor
- $v$ is the velocity of the conductor relative to the magnetic field.
Static EMF ( EMF in a battery)
$$
\mathcal{E}=V+I \cdot r
$$
where,
- $V$ is the terminal voltage of the battery
- $I$ is the current flowing through the circuit
- $r$ is the internal resistance of the battery
What is the SI Unit of Electromotive Force?
What is the SI unit of EMF? Let's have a look at what the unit of EMF is. The electromotive force formula is as follows:
$$
\mathcal{E}=V+I \cdot r
$$
substituting units of each term,
$
[\mathrm{V}]=[\mathrm{V}]+(\mathrm{A} \times \Omega)
$
Using ohm's law, $[\mathrm{V}]=[\mathrm{V}]+[\mathrm{V}]$
Hence, the unit of electromotive force is a volt.
Dimensional Formula Of EMF
Substituting dimensions of the terms in the equation,
$[\mathcal{E}]=\left[M^1 L^2 T^{-3} A^{-1}\right]+\left(\left[A^1\right] \cdot\left[M^1 L^2 T^{-3} A^{-2}\right]\right)$
Simplifying we get
$[\mathcal{E}]=\left[M^1 L^2 T^{-3} A^{-1}\right]+\left[M^1 L^2 T^{-3} A^{-1}\right]$
Hence the dimension of emf is
$$
[\mathcal{E}]=\left[M^1 L^2 T^{-3} A^{-1}\right]
$$
Difference Between EMF and Terminal Voltage
EMF and terminal voltage differences are as follows:
The potential difference between the terminals of a load when the circuit is on is known as terminal voltage. EMF, on the other hand, is defined as the highest potential difference delivered by the battery while no current is flowing.
The terminal voltage is measured with a voltmeter, while the EMF is measured with a potentiometer.
Is it possible for the electromotive force to be negative?
Yes, there is a possibility that the electromotive force is negative. Consider the case of an inductor that generates an EMF that is in opposition to the incoming power. The resulting EMF is thus interpreted as negative because the flow direction is opposite to that of the true power. As a result, the electromotive force is possible to be negative.
Difference Between Electromotive Force and Potential Difference
|
Electromotive Force
|
Potential Difference
|
| The work done on a unit charge is defined as EMF. | The energy dissipated when the unit charge passes through the components is defined as the potential difference. |
| The EMF does not change. | The difference in potential is not constant. |
| The EMF is unaffected by circuit resistance. | The resistance between the two points in a circuit during the measurement determines the potential difference. |
| E or $\varepsilon$ is the symbol for it. | The potential difference symbol is V. |
Potential Difference
The amount of work done in transporting a positive unit charge from one location to the other target point is described as the potential difference between two points in an electric circuit.
Work done is divided by the amount of charge transported equals the potential difference.
$\mathrm{V}=\frac{\mathrm{W} }{ \mathrm{q}}$
Here, the Potential Difference symbol is V
Example
When we state that the potential difference between two points is 3 volts, we mean that it takes 3 joules of energy to move a positive unit charge between them.
Terminal Voltage
Terminal voltage definition is when a current is supplied, the terminal voltage is the actual potential difference across the supply terminals.
Note:
When electrons move from negative to positive terminals in a closed circuit, the terminal voltage falls below the EMF value.
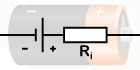
Internal Resistance
Chemical reactions within the cell can't keep up with the pace of charge separation to maintain maximal charge separation. Charges must flow between the electrolyte and the terminals, and there is always some internal resistance to this $(r)$.
As a result, there is an internal voltage drop equal to $Ir$ while current flows and the terminal voltage formula is given by $\mathrm{V}_{\mathrm{T}}=\varepsilon-\mathrm{Ir}$.
Note that when current $I=0$, the terminal voltage is equal to $\varepsilon$.
Here is a representation of the voltage source and internal resistance for your better understanding.
Factors Affecting EMF
- Rate of change of magnetic flux
- Number of turns in the coil
- Magnetic flux density
- Area of the coil
- Velocity of conductor
Types of EMF
Primary EMF or Static EMF
- It is generated by the difference in electric potential
- Battery and thermocouples are examples of static EMF
Induced EMF
- It is generated by the relative motion between the conductor and the magnetic field.
- Two types of induced EMF: motional EMF and transfer EMF
Also read:
Frequently Asked Questions (FAQs)
Full form of EMF is electromotive force.
SI unit of emf is Joules/Coulomb
Correct option is A.
Correct option is A.
IR = ε – Ir
I= R+r
I=1248
I = 0.25 A
Also Read
29 Nov'24 09:48 AM
23 Nov'24 01:56 PM
20 Nov'24 10:32 AM
17 Nov'24 10:02 AM
17 Nov'24 09:56 AM
14 Nov'24 07:31 PM
14 Nov'24 05:59 PM
14 Nov'24 01:16 PM
14 Nov'24 12:54 PM