Electrostatics - Coulomb's Law of Electrostatic
Have you ever tried to stick a balloon to a wall after rubbing it on your hair? So you might be curious about what Force is acting between the wall and the balloon. This powerful force is Electrostatic Force. By rubbing the balloon on your hair, you’re transferring tiny electric charges to it, making it attract to the wall, almost like a magnet. From printers to radar, everything involves Electrostatic force. In this article, we will explore the concept of Electrostatic force in detail.
JEE Main/NEET 2027: Physics Important Formulas for Class 10
NEET 2025: Mock Test Series | Syllabus | High Scoring Topics | PYQs
JEE Main: Study Materials | High Scoring Topics | Preparation Guide
JEE Main: Syllabus | Sample Papers | Mock Tests | PYQs
- What is Electrostatic Force?
- Applications of Electrostatic Forces
- Coulomb's law of electrostatic:
- Properties of Electrostatic Force

What is Electrostatic Force?
The force acting between two stationary charged particles is called an Electrostatic force. These charged particles can either pull the object toward each other (if the charges are opposite) or push the object away (if the charges are similar).
S.I. Unit of Electrostatic Force
The S.I. unit of electrostatic force is the Newton (N).
Applications of Electrostatic Forces
Some of the uses of Electrostatic force are as follows:
1. In electrostatic loudspeaker.
2. In electrostatic sprinkling of paints and powder coating.
3. In flyash collection in chimneys.
4. In a Xerox copying machine.
5. In the designing of a cathode-ray tube that is used in television and radar.
Also read
Coulomb's law of electrostatic:
Coulomb's law states that the attractive or repulsive forces amongst two fixed-point charges are:
- Directly proportional to the product of the magnitudes of the charges, and
- Inversely proportional to the square of the distance between them. This force acts along the line relating the two charges.
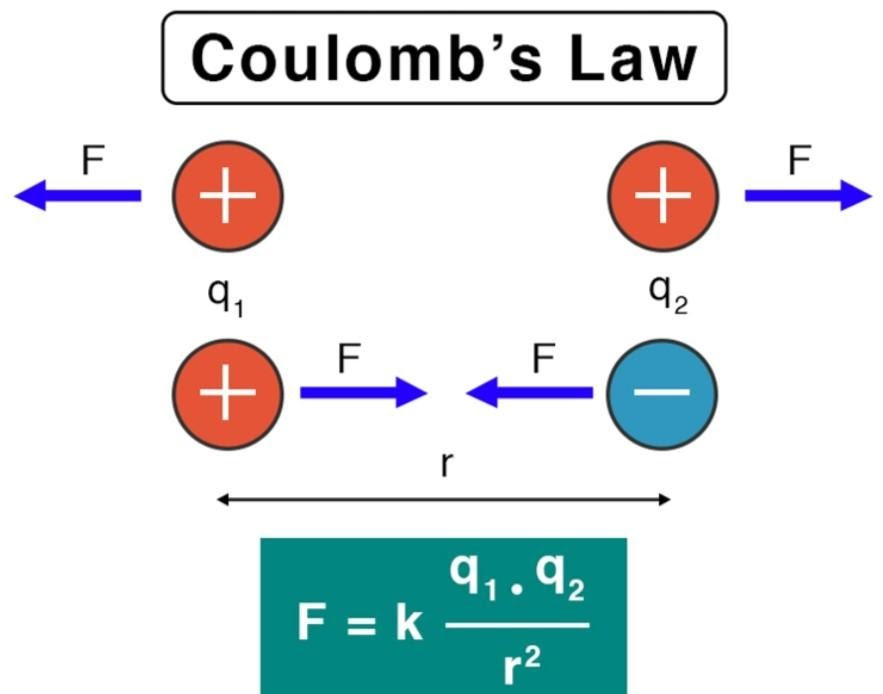
$$
F=\frac{k \cdot\left|q_1 \cdot q_2\right|}{r^2}
$$
where:
- $F=$ Electrostatic force between the charges (in Newtons, N)
- $k=$ Coulomb's constant $\left(8.9875 \times 10^9 \mathrm{~N} \mathrm{~m}^2 / \mathrm{C}^2\right)$
- $q_1$ and $q_2=$ Magnitudes of the two charges (in Coulombs, C)
- $r=$ Distance between the centers of the two charges (in meters, m )
It is a law that governs electrostatic force.
|
Related Topics |
Properties of Electrostatic Force
Attractive or Repulsive: Electrostatic Force can be either repulsive or Attractive in nature, depending on the charges involved.
- If the charges are similar (both positive or both negative) then repulsion will take place.
- If the charges are opposite (one positive and one negative) then attraction will take place.
Distance Dependent: Electrostatic force is inversely proportional to the distance between the charges as per the Coloumb's law. This means that force decreases as the distance between the charges increases.
Obeys Newton's Third Law: Electrostatic forces between two charges are equal in magnitude and opposite in direction. If one charge exerts a force on another, the second charge exerts an equal and opposite force on the first.
Conservative Force: It means that work done to move a charge from one point to another will depend only on the final and initial position, and remain independent on the path taken. This can be understood by learning the concept of Conservative and Non-conservative forces.
Magnitude Proportional to Charge: The electrostatic force is directly proportional to the product of the magnitudes of the charges involved. Larger charges result in a stronger force.
Frequently Asked Questions (FAQs)
Electrostatic is a branch of physics which works with the static electric charges.
In making loudspeaker and in sprinkling of paints and powder coating.
Unit of electrostatic force constant = N m2 C-2
Alike charges repels and unlike charges attracts each other.
Charles Augustin Coulomb
Also Read
16 Nov'24 12:54 AM
14 Nov'24 02:18 PM
14 Nov'24 01:05 PM
14 Nov'24 12:05 PM
14 Nov'24 10:56 AM
12 Nov'24 10:01 PM
12 Nov'24 07:54 PM
24 Sep'24 04:48 PM