Heat Transfer By Radiation
Have you ever felt the warmth of the sun on your skin even though you were standing in the shade? This experience is a direct result of heat transfer by radiation. Unlike conduction and convection, radiation does not need any medium to transfer heat. It's what enables us to feel the heat from the sun despite the vast vacuum of space between us.
This Story also Contains
- Heat Transfer by Radiation
- Prevost Theory of Heat Exchange
- Solved Example Based On Heat Transfer By Radiation
- Summary

In this article, we will cover the concept of Heat Transfer By Radiation. This concept falls under the chapter Properties of Solids and Liquids which is a crucial chapter in Class 11 physics. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more. Over the last ten years of the JEE Main exam (from 2013 to 2023), a total of one question has been asked on this concept. And no direct question in NEET from this concept.
Heat Transfer by Radiation
The process of the transfer of heat from one place to another place without any requirement of the medium is called radiation. It means that the radiation does not need any material medium to propagate.
Characteristics of Radiation
The process of the transfer of heat from one place to another place without heating the medium is called radiation.
Characteristics of the radiation are given below:
- The wavelength of thermal radiation ranges from 7.8×10−7 m to 4×10−7 m. The radiation heat transfer belongs to the infrared region of the electromagnetic spectrum. That is why thermal radiations are also called infrared radiations.
- Everybody whose temperature is above zero Kelvin emits thermal radiation. Practically it is not possible to reach 0 Kelvin in a finite number of steps, so every material in this universe emits radiation.
- The intensity of thermal radiation is inversely proportional to the square of the distance of the point of observation from the source (I∝1d2)
- As it is an electromagnetic wave, it follows laws of reflection, refraction, interference, diffraction, and polarisation.
Radiation Pressure
When these thermal radiations fall on a surface they exert pressure on that surface, which is called Radiation pressure.
- Radiation spectrum is obtained by quartz or rock salt prism because these materials do not have free electrons and interatomic vibrational frequency is greater than the radiation frequency, hence they do not absorb heat radiations.
- Interaction of Radiation with Matter-
When thermal radiations (Q) fall on a body, they are partly reflected, partly absorbed and partly transmitted as shown in the below figure.
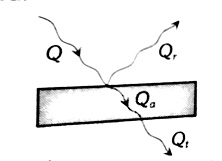
So we can write
Q=Qa+Qt+Qr or QQ=QaQ+QtQ+Qrr or 1=a+r+t
Where
QaQ=a= Absorptance QrQ=r= Reflectance QtQ=t= Transmittance
So
- If a = t = 0 and r = 1 then body is perfect reflector
If r = t = 0 and a = 1 then the body is a perfectly black body.
If, a = r = 0 and t = 1 the body is perfect transmitter
If t=0⇒r+a=1 or a=1−r
i.e. good reflectors are bad absorbers.
Prevost Theory of Heat Exchange
Everybody emits heat radiations at all finite temperatures (Except 0 K) as well as it absorbs radiations from the surroundings.
The amount of heat emitted/absorbed depends on the nature of the body, the temperature of the body and the cross-section of the body through which heat exchange is taking place.
The exchange of energy along various bodies takes place via radiation.
How the temperature of the body will vary will depend on the temperature of the surrounding
I. If surrounding temperature= body temperature
then Qemission =Qabsorbed
i.e the body will emit and absorb at the same rate
the temperature of the body remains constant (thermal equilibrium)
II. If surrounding temperature > body temperature
then Qemission <Qabsorbed
i.e. temperature of the body increases and it appears hotter.
III. If surrounding temperature < body temperature
then Qemission >Qabsorbed
i.e. temperature of the body decreases and consequently the body appears colder.
Recommended Topic Video
Solved Example Based On Heat Transfer By Radiation
Example 1: The intensity of radiation at a distance d from the source is
1) proportional to d
2) Inversely proportional to d
3) Inversely proportional to d2
4) proportional to d2
Solution:
The intensity of the radiation is given by :
Iα1d2
Hence, the answer is option (3).
Example 2: Q amount of heat falls on a surface. 20% heat is absorbed and 35% is reflected, then the transmittance is:
1) 0.35
2) 0.2
3) 0.45
4) 0.55
Solution:
When thermal radiations (Q) fall on a body, they are partly reflected, partly absorbed and partly transmitted as shown in the below figure.

So we can write
Q=Qa+Qt+Qr or QQ=QaQ+QtQ+Qrr or 1=a+r+t
Where
QaQ=a= Absorptance QrQ=r= Reflectance QtQ=t= Transmittance Qa=0.20QQr=0.35QQt=Q−Qa−Qr=0.45Qt=QtQ=0.45
Hence, the answer is option (3).
Example 3: If an amount of heat falls on the surface of a metal and 80% of the heat is reflected, then absorptance is:
1) 0.8
2) 0.2
3) 0.4
4) 0.6
Solution:
Absorptance is given by :
QaQ=a
where,
Q= thermal radiation Qa=Q−Qr=Q−0.8Q=0.2Qa=QaQ=0.2
Hence, the answer is option (2).
Example 4: Which of the following is incorrect for perfectly reflecting surface
1) a = 0
2) t = 0
3) r = 1
4) a = 1
Solution:
The radiant energy per unit area per unit time-
Qincident =Qabsorbed +Qreflected +Qtransmitted Qi=Qa+Qr+Qt1=QaQi+QrQi+QtQia=QaQir= absorptive power t=QrQi= reflective power ⇒a+r+t=1
For a perfectly reflecting body: reflective power r will be 1.
Since the body reflects all the radiation falling on the body.
⇒a=t=0
For a perfectly reflecting surface,
a=0,t=0&r=1
Hence, the answer is option (4).
Example 5: Which of the following relation between absorbance, transmittance and reflectance is correct?
1) a+r+t=Q
2) a+r+t=0.8
3) a+r+t=1
4) a+r+t=100
Solution:
When Thermal Radiation(Q) falls in a body -
Q=Qa+Qt+QrQQ=QaQ+QtQ+Qrr
1 = a + r + t
- wherein

Hence, the answer is option (3).
Summary
Heat transfer by radiation is a flow of energy via electromagnetic waves, predominantly in the infrared spectrum. All objects radiate according to their temperature. The hotter, the more energy they radiate. Since this is a non-medium-dependent process, it can take place through a vacuum. That's why we feel the heat from the sun. Some of the key concepts of this chapter are blackbody radiation, emissivity, and the Stefan-Boltzmann law which quantifies just how much power an object radiates. These include the greenhouse effect thermal insulation, and periodic energy-efficient building designs. The understanding of radiation helps in tapping the potential of solar energy and improved thermal imaging techniques for better climate modelling.
FAQ's
How is heat transferred by radiation?
Heat gets transferred by radiation as a process in which energy flows by directly transferring it through electromagnetic waves, even through a vacuum.
What is a blackbody in relation to radiation?
A blackbody is an idealised object which would, crossing the boundary from outside inward, perfectly absorb all incident radiation and, going outward, perfectly emanate it. It is used for comparison with thermal radiation.
Why are colour objects hotter in the sun than light-colour objects?
Dark colours absorb more radiation and hence transfer more energy to heat, while light colours reflect more radiation and absorb less.
Can radiation take place in a vacuum?
Yes, radiation can occur in a vacuum because it doesn't require any medium; it transfers energy through electromagnetic waves.
What is emissivity?
Emissivity is the relative ability of an object to give off radiation in comparison with a perfect blackbody. It ranges from 0 to 1.