Heat Transfer
Heat transfer is the process by which thermal energy moves from a region of higher temperature to a region of lower temperature. This fundamental concept plays a crucial role in various real-life applications, ranging from everyday tasks to complex industrial processes. Whether it's the warmth you feel from a cup of coffee, the cooling effect of an air conditioner, or the efficient operation of engines, heat transfer is constantly at work around us. There are three primary modes of heat transfer: conduction, convection, and radiation. For instance, when you touch a metal spoon in a hot soup, heat is conducted from the soup to your hand. Similarly, convection currents in the atmosphere help regulate climate, and radiation from the sun provides energy to Earth. Understanding heat transfer not only enhances our knowledge of physical phenomena but also helps in designing more efficient systems for energy utilization and thermal management in everyday life.
This Story also Contains
- Heat Transfer
- Conduction
- Convection
- Radiation
- Solved Examples Based on Heat Transfer
- Summary
.jpg)
Heat Transfer
Heat transfer is the movement of thermal energy from one object or substance to another due to a temperature difference. This process is essential in both natural and engineered systems, affecting everything from the weather patterns we experience to the design of heating and cooling systems in buildings. Heat transfer occurs through three primary mechanisms:
Conduction
This is the direct transfer of heat through a material, like when you touch a hot stove and feel the heat transfer to your hand. Metals are good conductors because heat moves easily through them.
Convection
This happens in fluids (liquids and gases) when warmer areas of a fluid rise and cooler areas sink, creating a cycle of heat movement. It’s how heat spreads in boiling water or why warm air rises in a room.
Radiation
This is heat transfer through electromagnetic waves, such as the warmth you feel from the sun on a clear day. No physical medium is required for radiation, making it different from conduction and convection.
Recommended Topic Video
Solved Examples Based on Heat Transfer
Example 1: The layer of the atmosphere is heated through
1) Convection
2) Conduction
3) Radiation
4) 2 & 3 both
Solution:
Natural Convection
This arises due to the difference of densities at two places.
wherein
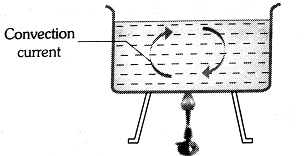
The heating of the atmosphere is mainly because of natural convection.
Hence, the answer is option (1).
Example 2: Heating of a room using a blower is an example of
1) Conduction
2) Natural convection
3) Forced convection
4) Radiation
Solution:
Forced Convection
If a fluid is forced to move to take up heat from a hot body.
wherein
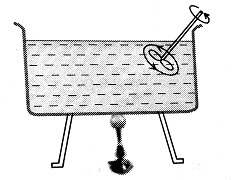
So, heat transfer with the help of a blower is an example of heat transfer through forced convection.
Hence, the answer is option (3).
Example 3: The height of Victoria Falls is $63 \mathrm{~m}$. What is the difference in temperature of water at the top and at the bottom of the fall?
$
\left[\text { Given } 1 \mathrm{cal}=4.2 \mathrm{~J} \text { and specific heat of water }=1 \mathrm{calg}^{-1 \circ} \mathrm{C}-1\right]
$
1) $1.476^{\circ} \mathrm{C}$
2) $14.76^{\circ} \mathrm{C}$
3) $0.147^{\circ} \mathrm{C}$
4) $0.014^{\circ} \mathrm{C}$
Solution:
As the water falls, all of it PE will continue into heat as it hits the bottom
$\begin{aligned} & m g h=m c \Delta T \\ & 10 \times 63=\frac{4.2 J}{10^{-3} \mathrm{~kg}{ }^0 \mathrm{C}} \times \Delta T \\ & \Delta T=\frac{630}{4.2}=\frac{6300}{42}=900 \times 10^{-3} \\ & \Delta T=150 \times 10^{-3} \\ & \Delta T \cong 0.15\end{aligned}$
Hence, the answer is option (3).
Example 4: If 5mg of a liquid at temperature T and specific heat 2s is mixed with 5mg of another liquid with temperature 2T and specific heat 3s then the final temperature of the mixture is
1) $\frac{2 T}{3}$
2) $\frac{8 T}{3}$
3) $\frac{8 T}{5}$
4) $\frac{3 C}{2}$
Solution:
If $m_1=m_2$
$
\theta_{m i x}=\frac{\theta_1 c_1+\theta_2 c_2}{c_1+c_2}
$
wherein
If bodies are of the same mass.
$
\begin{aligned}
& T^{m i x}=\frac{s_1 \theta_1+s_2 \theta_2}{s_1+s_2} \\
= & \frac{2 s T+3 s \cdot 2 T}{5 s} \\
= & \frac{8 T}{5}
\end{aligned}
$
Hence, the answer is option (3).
Example 5: The rate of radiation of the black body is E J/sec. The rate of radiation (in E) of this black body at 273oC will be :
1)16
2)8
3)4
4)1
Solution:
$
\begin{gathered}
E \propto \theta^4 \\
\Rightarrow E=\sigma \theta^4 \\
\frac{E_2}{E_1}=\left(\frac{T_2}{T_1}\right)^4
\end{gathered}
$
Where T is the Temperature in kelvin
$
\Rightarrow\left(\frac{273+273}{273+0}\right)^4=16
$
Hence, the answer is option (1).
Summary
Heat transfer is the process of how heat transfers from the hot object to the cold one. There are three ways of this happening: conduction, convection, and radiation. Conduction is when heat transfers through direct touch, convection is where heat is transferred through liquids or gases, and radiation is where heat is transferred in waves through space. Learning these routes helps us control heat in things like heaters, coolers, and so many other tools and gadgets.
