Heating Curve
The heating curve is a graphical representation that illustrates how a substance's temperature changes as it absorbs heat, moving through various states of matter—solid, liquid, and gas. This concept is not just confined to textbooks; it plays a crucial role in our everyday lives. Consider the process of boiling water to make tea: as you heat the water, it first warms up, then reaches its boiling point where it begins to evaporate without any increase in temperature until it turns into steam. This plateau in the temperature graph is a clear demonstration of the heating curve in action. Understanding this curve helps us comprehend how energy is used during phase changes, a fundamental concept in both science and practical applications like cooking, industrial processes, and even meteorology.
Heating Curve
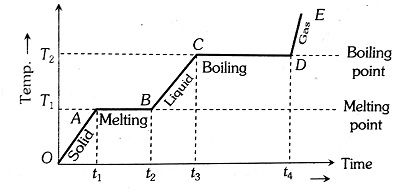
If a solid of mass (m) is heated at a constant rate such that it is undergoing a change of phase from solid to liquid and liquid to gas on a graph of Temperature and time is called the Heating curve.

Now we will discuss each phase one by one
(1) In the region OA, the temperature of a solid changes with time so, $Q=m c_S \Delta T \Rightarrow P \Delta t=m c_S \Delta T \quad[$ as $Q=P \Delta t]$
Here, P is the power and $\Delta t$ is the time interval.
So, from here we can deduce that $c_s \propto \frac{1}{\text { Slope of line } O A}$
Because this is the slope of OA
(2) Now come to the region AB, here temperature is constant, so it represents a change of phase, i.e., a change of phase from solid state to liquid state. Now you can see that between A and B substance is partly solid and partly liquid.
If $L_f$ is the latent heat of fusion $Q=m L_f$
$L_F=\frac{P\left(t_2-t_1\right)}{m} \quad\left[\right.$ as $\left.Q=P\left(t_2-t_1\right)\right]$
i.e., the Latent heat of fusion is proportional to the length of the line of zero slope. So, this line is parallel to the time axis.
(3) In the region BC temperature of liquid increases so, again $c_L \propto \frac{1}{\text { Slope of line } B C}$
Since it is sensible heat, the temperature will change in this zone BC.
(4) In the region CD temperature is again constant, so it represents the change of phase, i.e., boiling with boiling point T2. In this, the liquid is changing its phase from liquid to gas. The length of line CD is proportional to the latent heat of vaporisation (Lv)
Here, $Q=m L_v$
So, $L_v=\frac{P\left(t_4-t_3\right)}{m} \quad\left[\right.$ as $\left.Q=P\left(t_4-t_3\right)\right]$
So, $L_v \propto$ Length of line $C D$. It means that the line is parallel to the time axis.
(5) The line DE represents the gaseous state of the substance. Here, its temperature increases linearly with time. Just like solid and liquid, the reciprocal of the slope of the line will be proportional to the specific heat or thermal capacity of the substance in the vapour state.
We can understand better through video.
Solved Examples Based on Heating Curve
Example 1: Liquid oxygen at 450 K is heated to 3000 K at a constant pressure of 1 atm. The rate of heating is constant. Which one of the following graphs represents the variation of temperature with time
1) 
2)
3)
4)
Solution:
Phase
We use the term phase to describe a specific state of water. Such as solid liquid or gas.
wherein
At first, the temperature will increase then there will be a state change from liquid to gas.
The correct graph is represented by

Hence, the answer is the option (1).
Example 2: Latent heat of fusion (in cal/gm) of ice is
1) 1
2) 20
3) 60
4) 80
Solution:
The latent heat of the fusion of ice is 80 cal/gm.
Hence, the answer is the option (4).
Example 3: An ice cube of dimensions $60 \mathrm{~cm} \times 50 \mathrm{~cm} \times 20 \mathrm{~cm}$ is placed in an insulation box of wall thickness 1 cm The box keeping the ice cube at $0^{\circ} \mathrm{C}$ of temperature is brought to a room of temperature $40^{\circ} \mathrm{C}$ The rate of melting of ice is approximately:
(Latent heat of fusion of ice is $3.4 \times 10^5 \mathrm{~J} \mathrm{~kg}^{-1}$ and thermal conducting of insulation wall is $0.05 \mathrm{Wm}^{-10} \mathrm{C}^{-1}$)
1) $61 \times 10^{-3} \mathrm{~kg} \mathrm{~s}^{-1}$
2) $61 \times 10^{-5} \mathrm{~kg} \mathrm{~s}^{-1}$
3) $208 \mathrm{~kg} \mathrm{~s}^{-1}$
4) $30 \times 10^{-5} \mathrm{~kg} \mathrm{~s}^{-1}$
Solution:
The volume of ice cube $=6 \times 10^4(\mathrm{~cm})^3$
$$
=6 \times 10^{-2} \mathrm{~m}^3
$$

$\begin{aligned} & \text { Rate of fusion }=\frac{\mathrm{dQ}}{\mathrm{dt}}=\left(\frac{\mathrm{dm}}{\mathrm{dt}}\right) \mathrm{L}_{\mathrm{f}} \\ & \text { Rate of heat fiow }=\frac{\mathrm{KA}(\Delta \mathrm{T})}{\mathrm{t}} \\ & \mathrm{t} \rightarrow \text { thickness of wall } \\ & \text { At equilibrium, } \\ & \text { Rate of fusion }=\text { Rate of heat flow } \\ & \left(\frac{\mathrm{dm}}{\mathrm{dt}}\right) \mathrm{L}_{\mathrm{f}}=\frac{2 \mathrm{KA}_1 \Delta \mathrm{T}}{\Delta \mathrm{t}}+\frac{2 \mathrm{KA}_2 \Delta \mathrm{T}}{\Delta \mathrm{t}}+\frac{2 \mathrm{KA}_3 \Delta \mathrm{T}}{\Delta \mathrm{t}} \\ & \left(\frac{\mathrm{dm}}{\mathrm{dt}}\right) \times 3.4 \times 10^{\mathrm{S}}=\left(\frac{2 \times 0.05 \times 40}{10^{-2}}\right)\left[\mathrm{A}_1+\mathrm{A}_2+\mathrm{A}_3\right] \\ & \left(\frac{\mathrm{dm}}{\mathrm{dt}}\right)=\frac{4 \times 10^2}{3.4 \times 10^5} \times[3000+1000+1200] \times 10^{-4} \\ & =\frac{4}{3.4} \times 5.2 \times 10^{-4}=61 \times 10^{-5} \frac{\mathrm{kg}}{\mathrm{s}}\end{aligned}$
Hence, the answer is the option (2).
Example 4: A block of ice of mass 120 g at temperature $0^{\circ} \mathrm{C}$ is put in 300 g of water at $25^{\circ} \mathrm{C}$. The XG of ice melts as the temperature of the water reaches $0^{\circ} \mathrm{C}$. The value of X is___________ [Use specific heat capacity of water $=4200 \mathrm{Jkg}^{-1} \mathrm{~K}^{-1}$ Latent heat of ice $=3.5 \times 10^5 \mathrm{Jkg}^{-1}$ ]
1) 90
2) 80
3)100
4) 95
Solution:

$$
\begin{aligned}
& \mathrm{M}_1=120 \mathrm{~g} \\
& \mathrm{M}_2=300 \mathrm{~g}
\end{aligned}
$$
By the principle of calorimeter
$$
\begin{aligned}
& \text { Heat gain }=\text { Heat Lost } \\
& Q_1=Q_2 \\
& x L f=m_2 \Delta T_{\text {lost }} \\
& x \times 3.5 \times 10^5=300 \times 4200 \times 25 \\
& x=90 g
\end{aligned}
$$
Hence, the answer is the option (1).
Example 5: A steam engine intakes 50 g of steam at $100^{\circ} \mathrm{C}$ per minute and cools it down to $20^{\circ} \mathrm{C}$. If the latent heat of vaporization of steam is $540 \mathrm{cal} \mathrm{g}^{-1}$, then the heat rejected by the steam engine per minute is___________ $\times 10^3 \mathrm{cal}$.
(Given: specific heat capacity of water : $1 \mathrm{cal} \mathrm{g}^{-1{ }^{\circ} \mathrm{C}^{-1}}$)
1) 31
2) 23
3) 30
4) 29
Solution:
By the principle of the calorimeter,
Heat lost = Heat gained
In one minute,
$\mathrm{mL}_{\mathrm{v}}+\mathrm{mC} \Delta \mathrm{T}=$ Heat gained
$50 \times 540+50 \times 1 \times(80)=$ Heat gained
Heat gained $=$ Heat rejected by the steam engine
$=31000 \mathrm{cal}$
$=31 \times 10^3 \mathrm{cal}$
Hence, the answer is the option (1).
Summary
A heating curve indicates that a graph which is plotted for this scenario shows changes in the temperature of a substance against time while it is undergoing heating. It usually contains flat segments where the substance changes state—one at the melting and boiling points—and sloped ones in between, where the temperature increases. The flat parts of the graph show that energy is being used to alter the state, not to raise the temperature. Heating curves enable us to understand the relation of energy requirements for phase changes and various properties of substances.
