Isothermal Process - Definition, Example, Formula, FAQs
In this article, we will discuss the Isothermal Process which is a thermodynamic process in which the temperature of a system remains constant throughout the process. In real life, we can observe isothermal processes in refrigeration and air conditioning systems, where gases are compressed and expanded while maintaining a constant temperature to regulate cooling. Let's discuss the concept of the Isothermal process in detail
- What is an Isothermal Process?
- Isothermal Process Formula
- Work Done in the Isothermal Process
- PV Diagram for the Isothermal Process
- Isothermal Process Examples
- Solved Examples of Isothermal Process

What is an Isothermal Process?
An isothermal process is a type of thermodynamic process where the temperature of the system remains constant throughout the entire process. In this process, the system exchanges heat with its surroundings to maintain this constant temperature, despite changes in other properties like pressure or volume
What is Isotherm?
If we observe the relation between temperature and any other thermodynamic variable such as pressure, volume, and others and draw a graph on the Cartesian plane then, all the curves which represent two states of a system where the temperature is the same during an isothermal process are called isotherms.
So, Those curves or lines which represent two states of a system at the same temperature in an isothermal process are called an isotherm.
For example, a line drawn as shown in the diagram below has two states A and B and both are at the same temperature during an isothermal process so this line is called an isotherm. We can consider any other thermodynamic variable on the X-axis.
Also read -
- NCERT Solutions for All Subjects
- NCERT Notes For All Subjects
- NCERT Exemplar Solutions for All Subjects
Isothermal Process Formula
The basic formula in thermodynamics which shows that two states are in the isothermal process is simply written as
where,
- P represents the pressure and
- V represents the volume of an isothermal process in two states 1 and 2
Work Done in the Isothermal Process
When a system undergoes an isothermal process, either work is done on it or work is done by it and this work is different for different processes.
In the isothermal process work done by the system is calculated using the formula:
where,
- V represents volume at two different states being at a constant temperature of T and R is the universal gas constant.
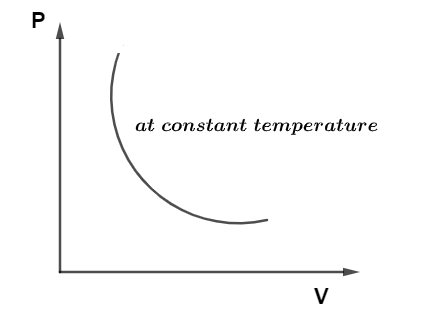
PV Diagram for the Isothermal Process
A diagram representation of the pressure and volume of an isothermal process on a cartesian plane is called a PV diagram for an isothermal process and it is best shown in the diagram given below:
Change in Internal Energy in the Isothermal Process
The internal energy of any thermodynamic system is calculated as
∆U=nCv∆T
where,
- n represents the total number of moles of a gas
- C represents the specific heat of the gas but at constant volume
- T represents temperature
Related Terms
In an isothermal process, the change in temperature is zero because of the constant temperature in the isothermal process. So, In an isothermal process, the change in internal energy is zero.
Recommended Topic Video
Isothermal Process Examples
- When ice melts at a temperature of zero degrees then the whole ice melts but the temperature of the system remains the same over the period of time, hence this is an example of an isothermal process.
- All the thermodynamic reactions which occur inside the refrigerator are isothermal processes because the temperature of the refrigerator remains the same.
- and, all such thermodynamic processes which are carried out at constant magnitude of temperature will be considered as isothermal processes.
Solved Examples of Isothermal Process
1- Which of the following statements correctly describes the relationship between the slope of the isothermal curve and the slope of the adiabatic curve for a gas process?
1. Slope of the isothermal curve = Slope of the adiabatic curve
2. Slope of the isothermal curve
3. Slope of the adiabatic curve
4. Slope of the adiabatic curve
Solution:
Differentiating
Hence, the slope of the isothermal curve is:
Differentiating
Hence, the slope of the adiabatic curve is:
From the equations above:
Hence, the answer is the option 3.
2- 2 moles of an ideal is isothermally expanded to 3 times its original volume at 300 K . Calculate the Work done and heat absorbed by the gas?
Given (
Solution:
Here
Now using the above equation
Substituting the above values, we get
Now heat absorbed
3. Draw the T-P diagram for the given P-V diagram for an ideal gas.

Solution:

So it means temperature is constant. So here is the TP diagram
Frequently Asked Questions (FAQs)
Since, processes which occur at constant temperature are called isothermal processes. Process which occurs at constant magnitude of volume is called Isochoric. And, all the thermodynamic processes which occur at constant heat are called adiabatic processes, So, the correct option is (C) Heat.
The thermodynamics process in which the temperature of the whole system remains constant over a period of time is called isothermal process. So, the constant temperature of any system makes the process an isothermal process. For example, melting of ice at zero degree is such an example of an isothermal process.
In an isothermal process, the temperature of the system remains the same, so the change in temperature is always zero, and due to this the change in internal energy in an isothermal process is always zero, SO, the correct option is (D) zero.
In an isothermal process, the temperature of the whole system is always the same and as well as the change in internal energy in isothermal process of the system is always zero. So, the correct option is During isothermal process (A) Temperature remains same and (B) Change in internal energy is zero.
Temperature remains constant.
Internal energy of the gas unchanged
Never

