Mayer's Formula
Mayer's formula explains the relationship between the specific heat of gas at constant pressure and constant volume, which is one of the most essential equations in thermodynamics. According to it, gas constant (R) is the difference between specific heat at constant pressure (Cp) and specific heat at constant volume (Cv). Thus, this makes it possible to understand better how energy flows take place in gases and how their heat capacity changes depending on the circumstances.
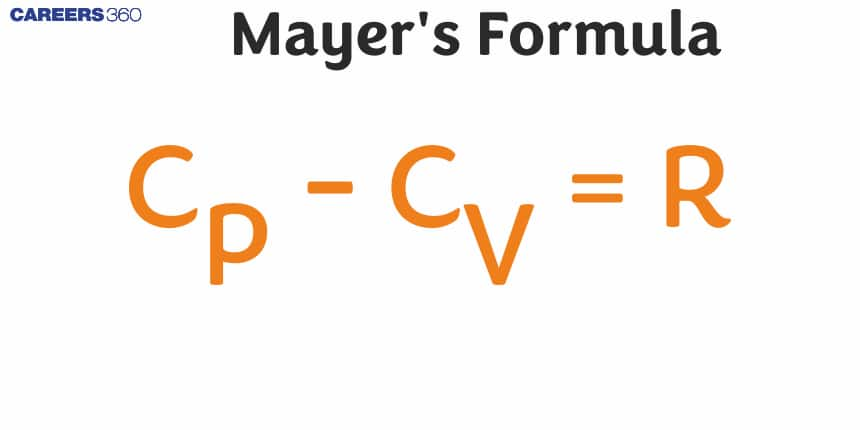
In this article, we will cover the concept of the 'Mayer's Formula’. It is not only essential for board exams but also for competitive exams like the Joint Entrance Examination (JEE Main), National Eligibility Entrance Test (NEET), and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more. Over the last ten years of the JEE Main exam (from 2013 to 2023), seventeen questions have been asked on this concept. And for NEET two questions were asked from this concept.
Let's read this entire article to gain an in-depth understanding of Mayer's Formula.
Mayer's formula
Molar Specific heat of the gas at constant volume
and Molar-specific heat capacity at constant pressure
Mayer's formula gives the relation between
or we can say that molar Mayer’s formula shows that specific heat at constant pressure is greater than that at constant volume.
Specific Heat in Terms of Degree of Freedom
1. Molar Specific heat of the gas at constant volume ($C_v$)
For a gas at temperature T, the internal energy
Also, as we know any gas heat supplied at a constant volume
From the equation (i) and (ii)
where
f = degree of freedom
R= Universal gas constant
Molar Specific heat of the gas at constant pressure ($C_p$)
From Mayer's formula, we know that
Atomicity or adiabatic coefficient ($\gamma$)
It is the ratio of
for Diatomic gas
for Triatomic gas
What is a Gaseous Mixture?
If two non-reactive gases
In the mixture
Then Specific heat of the mixture at constant volume will be
Similarly, the Specific heat of the mixture at constant pressure will be
And adiabatic coefficient ($\gamma$) of the mixture is given by
Also
Similarly, the Degree of freedom of mixture is given as
Similarly, the molar mass of the mixture
Learn Mayer's Formula With a Video Lecture
Solved Examples Based on Mayer's Formula
Example 1: The specific heats
1) A is rigid but B has a vibrational mode.
2) A has a vibrational mode but B has none.
3) A has one vibrational mode and B has two.
4) Both A and B have a vibrational mode each.
Solution:
Specific heat capacity at constant pressure -
wherein
For gas A,
3 translations, 2 rotations, Remaining vibrational mode
For gas B,
3 translations, 2 rotations, no vibrational mode
Hence, the answer is the option (2).
Example 2: Two moles of helium are mixed with n moles of hydrogen.
If
1) 1
2) 3
3) 2
4) 1.5
Solution:
As we have learned
Atomicity or adiabatic coefficient (gamma) -
wherein
for Monoatomic gas
for Diatomic gas
for Triatomic gas
or n=2
Hence, the answer is option (3).
Example 3: Consider a mixture of n moles of helium gas and 2n moles of oxygen gas (molecules taken to be rigid) as an ideal gas. Its Cp/Cv value will be :
1) 40/27
2) 23/15
3) 19/13
4) 67/45
Solution:
For monoatomic gas (He)
For diatomic gas
Hence, the answer is the option (3).
Example 4: Two moles of an ideal gas with
1) 1.50
2) 1.45
3) 1.47
4) 1.42
Solution:
For ideal gas:-
For first case:-
So,
For second case:-
Hence, the answer is the option (4).
Example 5: Two moles of helium gas are mixed with three moles of hydrogen molecules (taken to be rigid). What is the molar specific heat (in J/mol K) of the mixture at constant volume? (R=8.3 J/mol K)
1) 15.7
2) 17.4
3) 19.7
4) 21.6
Solution:
Hence the answer is the option (2).
Summary
Mayer’s Law is a basic equation in thermodynamics relating the specific heat capacities of a gas at constant pressure denoted by Cp and at a constant volume Cv. His law states that the difference between the specific heat capacity at constant pressure Cp and the specific heat capacity at constant volume is equal to the gas constant R. With this principle, we can be able to know how gases take in heat and release it under varying circumstances.
