Radioactive Decay - Meaning Types , FAQs
In this article, we define radioactive decay is, radioactive decay law, concept of decay constant, disintegration constant, types of radioactive decay, radioactive equilibrium, and the difference between radioactive equilibrium and chemical equilibrium.
What is radioactive decay?
Radioactive decay is a process by which the nucleus of an unstable atom loses by emitting radiation. It is also called nuclear decay, radioactivity, or nuclear disintegration.
Explain the decay process.
The decay process or radioactive process is a property of the nuclei of an atom. A material is called a radioactive or radioactive element if it contains unstable nuclei. By this process, the nucleus becomes more stable by emitting its energy. The mass and charge are conserved in the decay process.
Theory of radioactivity:
Radioactivity or the radioactive decay process involves the continuous emission of radiation by unstable nuclei in the form of radiation. The nuclei of the atom emit ionizing particles by losing their energy spontaneously. Due to the loss of energy, two types of atoms called parent nuclide and daughter nuclide are formed. The parent nuclide is the decaying nucleus and the daughter nuclide is obtained from the parent nuclide. Radioactive decay is called a nuclear transformation that yields a daughter nuclide that has protons or neutrons. This condition is not applicable in gamma decay, a type of radioactive decay. A new element is obtained as the number of protons in the atom changes.
Also read -
Types of radioactive decay:
Radioactive decay is classified into three types:
- Alpha decay: The nuclear decay process in which alpha particle is emitted is called alpha decay.
It is represented as, E=(mi-mf-mp)c2
Here, E is the energy, c is the speed of light, mi is the mass of nucleus before decay, mf is mass of nucleus after decay, and mp is the mass of the particle emitted in the decay process. Consider an example for the alpha decay of Th-230.
90230Th→24He+88226Ra
In this equation, thorium-230 is converted to radium-226 with the emission of an alpha particle.
- Beta-decay: In the decay process, if the beta particle is emitted then it is called beta decay. The nucleus emits a high-energy electron in the form of a beta particle. The beta particle is considered an electron as it is an electron ejected from the splitting of the neutron. An example for the beta decay of Th-234 is represented as:
90234Th→-10e+91234Pa
In the above equation, thorium-234 is converted to Pa-234 with the emission of a beta particle.
- Gamma decay: A nuclear disintegration in which gamma rays are emitted is called gamma decay. Consider the example of U-238.
92238U→24He+90234Th+200
In this equation, uranium-238 is converted to thorium-234 with the emission of gamma rays along with the alpha particle.
Related Topics Link, |
State radioactive decay law.
Radioactive decay law states that “the rate of radioactive disintegration is directly proportional to the number of nuclei of the elements present at that time”.
If N is the number of nuclei and dN is the number of radioactive decay per unit time dt then, the law is represented as,
dN/dt=N
dN/dt=-λN
In the above equation, λ is the radioactive constant.
Derive the law of radioactive decay.
From the radioactive decay equation,
dN/dt=-λN ……………………………. (1)
Consider at time t=0, the number of nuclei is N0 and at time t, the number of nuclei is N.
On integrating equation (1),
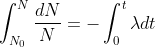
logeN-logeN0=-λt
loge(N/N0)=-λt
N/N0=e-λt
N=N0e-λt
The above equation is the formula for the radioactive decay law.
Also Read:
- NCERT solutions for Class 12 Physics Chapter 1 Electric Charges and Fields
- NCERT Exemplar Class 12 Physics Solutions Chapter 1 Electric Charges and Fields
- NCERT notes Class 12 Physics Chapter 1 Electric Charges and Fields
Rate of disintegration or rate of radioactive decay:
The rate of disintegration or rate of radioactive decay is the number of radioactive nuclei reducing per unit time. The rate of disintegration is denoted by(-dNt/dt).
What is the decay constant?
Decay constant lambda (λ) is the time taken by the nucleus of an atom to reduce half of its initial value. It is also defined as the reciprocal of the time in which the number of atoms in a radioactive element reduces to 36.8% of the initial value.
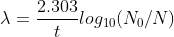
SI unit of the decay constant is s-1.
Radioactive disintegration series:
A radioactive nucleus undergoes a series of decay. In a radioactive disintegration series, the decay process is continued until the unstable nuclei become stable nuclei.
The half-life of a radioactive element:
The time taken by the radioactive element to reduce half of its initial value is called the half-life of a radioactive element. It is represented by T1/2.
T1/2=ln2/λ=0.693/λ
Also check-
- NCERT Exemplar Class 11th Physics Solutions
- NCERT Exemplar Class 12th Physics Solutions
- NCERT Exemplar Solutions for All Subjects
NCERT Physics Notes:
Frequently Asked Questions (FAQs)
Decay is the process in which an object gets decomposed.
The meaning of radioactive element is the element having unstable nuclei in its atom.
In the decay process, unstable nuclei that emit radiation are called radioactive nuclei.
The process in which radiation is emitted in the form of particles or photons of high energy is called radioactivity.
The decay constant is the reciprocal of the time by which the left number of nuclei in the element decreases to 1/e times the number of nuclei before the decay.
t=2.303log10(N0N)
Here λ is the decay constant.
The number of disintegration per second is the activity of the radioactive sample.
Decay of radioactive isotopes is the process of emitting radiation where the time period of the isotope is measured in half-life.
The law of radioactive disintegration is used in the calculation of decay rate.
