Thermal Expansion In Liquids And Gases
Consider a metal bridge during the hottest summer days. As the temperature rises, the metal will expand. At nightfall, with the cool, metal will contract. This process of expansion and contraction develops forces within the material and is called thermal stress and strain, respectively. Thermal stress and strain are important concepts that, through people, enable the design of safe, long-lasting structures. Knowing these concepts helps one to understand changes in temperature on materials and why it is important to accommodate such modifications on construction and everyday objects.
JEE Main 2025: Physics Formula | Study Materials | High Scoring Topics | Preparation Guide
JEE Main 2025: Syllabus | Sample Papers | Mock Tests | PYQs | Study Plan 100 Days
NEET 2025: Syllabus | High Scoring Topics | PYQs | Crack NEET in 2 months - Study Plan
- Thermal Expansion In Liquids And Gases
- Thermal Expansion in Liquids
- Solved Examples Based On Thermal Stress And Thermal Strain
- Summary

This concept is the part of chapter Properties of Solids and Liquids in Class 11 Physics. It is not only essential for board exams but also for competitive exams like the JEE Main, NEET and other entrance exams such as SRMJEE, BITSAT, WBJEE, BCECE and more. Over the last ten years of the JEE Main exam (from 2013 to 2023), one question has been asked on this concept one question has also been asked in the NEET exam from this concept.
Thermal Expansion In Liquids And Gases
Let's discuss one by one briefly
Thermal Expansion Liquid
The particles of liquid are less tightly packed when compared to solids. Liquids have the ability to take the shape of the container in which liquids are kept. The particles of liquid have less space between them to move so the compression liquids are difficult but not as in solids. The Volume of Liquid is fixed but the shape of Liquid is not fixed. The rate of Diffusion in liquids is higher as compared to solids. Example: water, milk, coffee, blood etc.
Thermal Expansion Gas
The particles of gas are far from each other. The force of attraction between the particles of gases is almost negligible and hence they can move independently. The volume and shape of the gas are not fixed. The particles of gas have more space between them to move so the compression gases are easy. The rate of diffusion is higher as compared to solids and liquids. Examples: air, oxygen, nitrogen, carbon dioxide, etc.
Thermal Expansion in Liquids
Like solids, liquids do not have linear and superficial expansion but liquid only undergoes volume expansion.
We always need some solid vessel to keep the liquid, so liquids are always to be heated along with a vessel which contains them so initially on heating the system (System is liquid + vessel here). Initially, the level of liquid in the vessel falls (vessel expands more since it absorbs heat and liquid expands less) as the volume expansion coefficient of solid is more than that of liquid but later on, it starts rising due to faster expansion of the liquid (because now solid transfer all the heat to liquid and that is the condition of steady-state)

So, from above we can conclude that the actual increase in the volume of the liquid = The apparent increase in the volume of liquid + the increase in the volume of the vessel.
Basically, liquids have two coefficients of volume expansion -
Co-efficient of apparent expansion
Co-efficient of real expansion
Also coefficient of expansion of flask
So,
So the change (apparent change) in volume in liquid relative to the vessel is -
Where,
Anomalous expansion of water: Generally any material expands on heating and contracts on cooling. But in the case of water, it expands on heating if its temperature is greater than 4°C. In the range 0°C to 4°C, water contracts on heating and expands on cooling, i.e.
This is the anomalous behaviour of water which causes ice to form first at the surface of a lake in cold weather. So, as winter approaches, the water temperature increases initially at the surface. It results in the water sinking because of its increased density. Consequently, the surface reaches 0°C first and because of that the lake becomes covered with ice. This property of water makes the aquatic life survive the cold winter as the lake bottom remains unfrozen at a temperature of about 4°C.
At 4°C, the density of water is maximum while its specific volume is minimum.

Variation of Density with Temperature -
Most substances (solid and liquid) expand heat is supplied to them, i.e., the volume of a given mass of a substance increases on heating, so the density should decrease
So,
Here,
Expansion of Gases
As we know the gases have no definite shape. It takes the shape of the vessel in which it is kept. Therefore gases have only volume expansion. Since the expansion of the container (Because the container is solid) is negligible in comparison to the gases, therefore gases have only real expansion.
Coefficient of volume expansion: At constant pressure, the unit volume of a given mass of a gas, increases with a 1°C rise of temperature, which is called the coefficient of volume expansion.
Coefficient of pressure expansion :
Recommended Topic Video
Solved Examples Based On Thermal Stress And Thermal Strain
Example 1: When a liquid in a glass vessel is heated its apparent expansion is
1)
2)
3)
4)
Solution: As we learned
Co-efficient of Apparent Expansion -
Example 2: A glass flask of volume 1 litre is fully filled with mercury at
1) 15.2
2) 17.2
3) 19.2
4) 21.2
Solution:
Co-efficient of Real Expansion flask -
Example 3: A liquid with a coefficient of volume expansion
1)
2)
3)
4)
Solution:
As we learned
Thermal Expansion in Liquids -
Liquid have only volume expansion.
- wherein
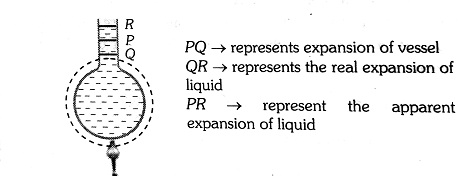
Since the liquid overflows, the volume expansion of the liquid is more than that of the material of the container.
Example 4: The level of liquid kept in a vessel will fall on heating if
1)
2)
3)
4) none of the above
Solution:
As we know,
Expansion (Real gamma less than gamma vessel) -
wherein
The level of liquid in the vessel will fall on heating.
Example 5: The real coefficient of expansion of liquid is 5 times the volume coefficient of expansion of the vessel. The ratio of real and apparent expansion of the liquid is
1)
2)
3)
4)
Solution:
Summary
Thermal stresses are created in a material due to temperature changes and its restraint from expansion or contraction, resulting in internal forces; thermal strain is deformation caused by such stresses. All materials expand upon heating and contract upon cooling. That is, if this movement is restrained, it creates stress.
Also Read
02 Dec'24 12:36 AM
21 Nov'24 01:48 PM
20 Nov'24 10:28 AM
18 Nov'24 05:26 PM
12 Nov'24 11:55 PM
12 Nov'24 11:20 PM
12 Nov'24 09:27 PM
25 Sep'24 06:28 PM
25 Sep'24 11:47 AM
24 Sep'24 09:56 PM