To Study The Effect Of Detergent On Surface Tension Of Water By Observing Capillary Rise
In this experimental work, soaps’ effects on water surface tension will be examined by measuring the capillary/micropipette rise/drive-up of liquids. This crucial/life-saving action feature enables liquids to withstand outside forces thus contributing to a lot great extent to the countless natural processes and industries. Therefore, should we observe water rising up through a narrow tube upon the addition of various quantities of soap, then we can simply find out/deduct the way that such substances interfere with this main characteristic.
This Story also Contains
- Aim
- Apparatus
- Theory
- Diagram
- Procedure
- Observation
- Solved Examples Based on the Study Effect of Detergent on Surface Tension of Water by Observing Capillary Rise
- Summary
It is important to inspect capillary rise in water and while conducting such investigation, learn its surface tension modification. This area is vital for a basic understanding of liquid dynamics as one may appreciate how interaction with surfaces plus other materials depends on surface tension. Understanding molecular interactions between liquid molecules changes our awareness of how detergent affects this fundamental property.
Aim
To study the effect of detergent on surface tension of water by observing capillary rise.
Apparatus
Three capillary tubes of different radii and a tipped pointer clamped in a metallic plate with a handle, travelling microscope, clamp and stand, a fine motion adjustable height stand, a flat bottom open dish, clean water in a beaker, and thermometer.
Theory
A detergent when added to distilled water reduces the surface tension of water. If we use the same capillary tube to study the rise of pure distilled water and then the rise of detergent mixed water (solution), we shall find that
the rise will be less in the case of solution. If the quantity of detergent (solution concentration) is increased, the rise will
be still lesser.
T=(h+r3)rρg2cosθ but h>>rT=rρhg2cosθT∝h
Diagram
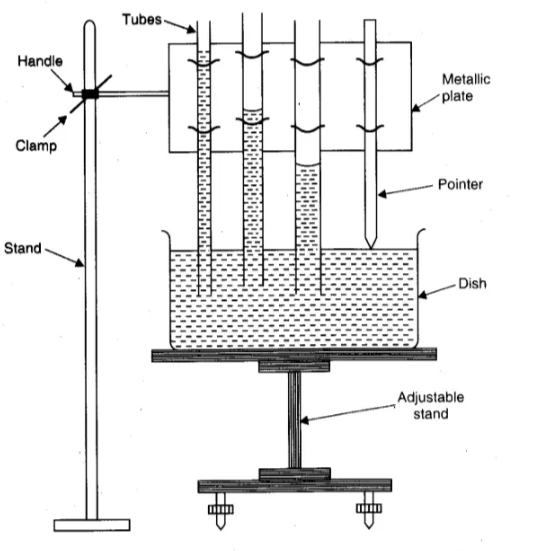
Procedure
(a) Setting the apparatus
1. Place the adjustable height stand on the table and make its base horizontal by level-ling screws.
2. Take dirt and grease-free water in an open dish with a flat bottom and put it on the top of the stand.
3. Take three capillary tubes of different radii (ranging from 0.05 mm to 0.15 mm ).
4. Clean and dry them, and clamp the capillary tubes in a metallic plate in order of increasing radius. Also, clamp a pointer after the third capillary tube.
5. Clamp the horizontal handle of the metallic plate in a vertical stand, so that the capillary tubes and the pointer become vertical.
6. So adjust the height of the metallic plate that the capillary tubes dip in water in an open dish.
7. Adjust the position of the pointer, such that its tip just touches the water surface.
(b) Measurement of capillary rise
8. Find the least count of the travelling microscope for the horizontal and vertical scales. Record the same in the notebook.
9. Raise the microscope to a suitable height, keeping its axis horizontal and pointing towards the capillary tubes.
I0. Bring the microscope in front of the first capillary tube (which has maximum rise).
11. Make the horizontal crosswire just touch the central part of the concave meniscus seen convex through a microscope.
12. Note the reading of the position of the microscope on the vertical scale.
13. Now move the microscope horizontally and bring it in front of the second capillary tube.
14. Lower the microscope and repeat steps 11 and 12.
15. Repeat steps 11 and 12 for the third capillary tube.
16. Lower the stand so that the pointer tip becomes visible.
17. Move the microscope horizontally and bring it in front of the pointer.
18. Lower the microscope and make the horizontal cross wire touch the tip of the pointer. Repeat step 12.
(c) Measurement of the internal diameter of the capillary tube
19. Place the first capillary tube horizontally on the adjustable stand.
20. Focus the microscope on the end dipped in water. A white circle (inner bore) surrounded by a green circular strip (glass cross-section) will be seen.
21. Make a horizontal cross-wire touch the inner circle at A. Note microscope reading on a vertical scale.
22. Raise the microscope to make the horizontal cross-wire touch the circle at B. Note the reading (the
difference gives the vertical internal diameter AB of the capillary tube).
23. Move the microscope on a horizontal scale and make the vertical cross wire touch the inner circle at C.
Note microscope reading on the horizontal scale.
24. Move the microscope to the right to make the vertical cross-wire touch the circle at D. Note the reading
(the difference gives the horizontal internal diameter CD of the capillary tube).
25. Repeat steps 19 to 24 for the other two capillary tubes.
26. Note the temperature of the water in the dish.
27. Record your observations as given ahead.
28. Take a known volume of distilled water from the same sample.
29. Dissolve a small known mass of a detergent in the water to make a dilute solution.
30. Find the rise of the solution in a capillary tube. The rise will be less than for pure water.
31 Add the double mass of detergent in the same volume of water to have a solution with a double concentration.
32. Find the rise of this concentrated solution in the same capillary tube. The rise will be still lesser.
33. Repeat with the solution of the same detergent having increased concentration. The rise will decrease as concentration increases.
Observation
The rise in the capillary tube decreases with the addition of detergent in pure water. With more addition of detergent, rise becomes lesser and lesser.
Result
The detergent reduces the surface tension of water.
Precautions
1. Capillary tube and water should be free from grease.
2. The capillary tube should be set vertically.
3. Microscope should be moved in a lower direction only to avoid backlash errors.
4. The internal diameter of the capillary tube should be measured in two mutually perpendicular directions.
5. Temperature of water should be noted.
Solved Examples Based on the Study Effect of Detergent on Surface Tension of Water by Observing Capillary Rise
Example 1: The amount of energy (μJ) released when eight droplets of mercury (surface tension 0.55 Nm-1 ) of radius 1mm each combine into one drop is
1) 27.6
2) 24.2
3) 16.8
4) 18.7
Solution:
T=0.55 N/m
Amount of energy evolved =W=T⋅ΔA
∴W=055×ΔAΔA=(n×4πr2)−(1×4πr2)
where r is the radius of a small drop. R is the radius of the big drop.
=(4π8r2)−4πR2ΔA=4π(8r2−R2)
Also as mass remains the same hence ρ×43πr3×8=ρ×43πR3
∴r3=(R3/8)∴r=(R/2) i.e. R=2rΔA=4π(8r2−4r2)− from ΔA=16πr2 from (1)W=0.55×16π×(10−3)2=27.6×10−6 J=27.6μJ
Hence, the answer is the option (1).
Example 2: What happens to the capillary rise of a liquid when the radius of the capillary tube increases?
1) Remain unchanged
2) Become zero
3) Increases
4) Decreases
Solution:
To study the effect of detergent on the surface tension of water by observing the capillary rise
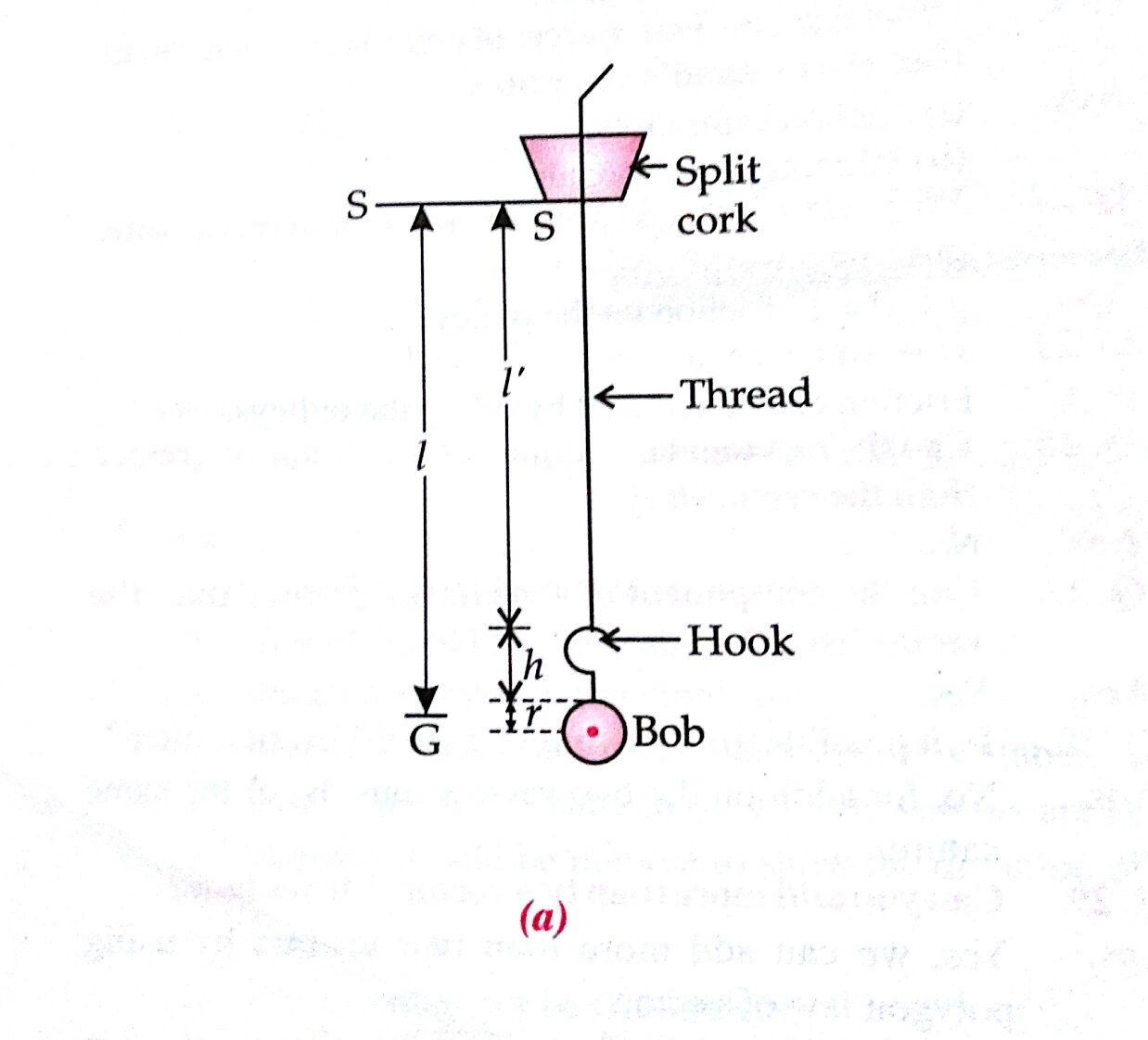
T=[rρhg2cosθ]
Tah
h=2scosΘρrg∴hα1r
If the radius of the capillary tube increases then its height will decrease.
Hence, the answer is the option (4).
Example 3: A glass capillary tube with a radius of r=0.5 mm is dipped into a container of water. The water rises to a height of h=3.0 cm inside the capillary due to capillary action. Calculate the surface tension of water using the given data. If a detergent is added to the water, reducing the surface tension to 2/3 of its original value, calculate the new capillary rise.
1) 0.05 m
2) 0.04 m
3) 0.01 m
4) 0.02 m
Solution:
Given:
- The radius of the capillary tube r=0.5 mm=0.5×10−3 m
- Capillary rise h=3.0 cm=3.0×10−2 m
- Reduced surface tension due to detergent T′=23×T where T is the original surface tension.
Step 1: Calculate the original surface tension T using the capillary rise formula:
T=4×h×rπr2T=4×3.0×10−2×0.5×10−3π×(0.5×10−3)2T=0.120 N/m
Step 2: Calculate the new surface tension T′ due to the detergent:
T′=23×0.120 N/mT′=0.080 N/m
Step 3: Calculate the new capillary rise h′ using the reduced surface tension T′ :
h′=T′4×rh′=0.080 N/m4×0.5×10−3 mh′=0.040 m
The surface tension of water (T) is 0.120 N/m. After adding the detergent, the new capillary rise (h′) is 0.040 m.
Hence, the answer is the option (2).
Example 4: A glass capillary tube with a radius of r=0.2 mm is dipped into a container of liquid mercury. The liquid mercury falls to a height of h=3.0 mm below the normal liquid level inside the capillary due to capillary action. Calculate the surface tension of mercury using the given data. If a detergent is added to the mercury, increasing the surface tension to 1.5 times its original value, calculate the new capillary fall.
1) 0.05 m
2) 0.0895 m
3) 0.06 m
4) 0.002 m
Solution:
Given:
- The radius of the capillary tube r=0.2 mm=0.2×10−3 m
- Capillary fall h=3.0 mm=3.0×10−3 m
- Increased surface tension due to detergent T′=1.5×T, where T is the original surface tension
Step 1: Calculate the original surface tension T using the capillary fall formula:
T=4×h×rπr2T=4×3.0×10−3×0.2×10−3π×(0.2×10−3)2T=4.772×10−2 N/m
Step 2: Calculate the new surface tension T′ due to the detergent:
T′=1.5×4.772×10−2 N/mT′=7.158×10−2 N/m
Step 3: Calculate the new capillary fall h′ using the increased surface tension T′ :
h′=T′4×rh′=7.158×10−2 N/m4×0.2×10−3 mh′=0.0895 m
The surface tension of mercury (T) is 4.772×10−2 N/m. After adding the detergent, the new capillary fall (h′) is 0.0895 m .
Hence, the answer is the option (2).
Example 5: An experiment is conducted to determine the speed of sound (v) in air using a resonance column. The column is filled with a known liquid, and a tuning fork of known frequency (f) is used. The first resonance occurs at a column height (h) of 25 cm, and the second resonance occurs at 75 cm. Calculate the speed of sound in the air.
1) 100 m/s
2) 235 m/s
3) 169 m/s
4) 128 m/s
Solution:
Given values:
- Frequency of tuning fork (f)=256 Hz
- First resonance height (h1)=25 cm
- Second resonance height (h2)=75 cm
The speed of sound ( v ) can be calculated using the formula for the fundamental frequency of an open-open resonance column:
v=2h1f
Step 1: Calculate the speed of sound (v) using the first resonance height and frequency:
v=2×25 cm×256 Hz
Step 2: Calculate v:
v=12800 cm/s
The speed of sound in air is 12800 cm/s.
Step 3: Convert the speed of sound to m/s.
v=128 m/s
The speed of sound in air is 128 m/s.
Hence, the answer is the option (4).
Summary
The experiment studies the influence of detergents on water surface tension by measuring capillary elevation. Water surface tension is the pull that makes water form droplets and rise in capillaries. The addition of detergent to water will result in lower surface tension because these molecules of detergent break off hydrogen bonds between these molecules of water. To measure the levels that the water increases on a capillary tube when there is no detergent as well as after adding it is the aim of this project.
