SMBS Full Form
What is the full form of SMBS?
SMBS stands for Sodium metabisulfite. The chemical formula for Sodium metabisulfite is \mathrm{N{{a}_{2}}S_2{{O}_{5}}} ![]() , and its molecular weight is 190.107g/mol. It is an ionic compound of Sodium that contains Sulphur. Sodium metabisulfite is soluble in both water and glycerol. The positive charge of sodium contrasts with the negative charge of metabisulfite. Having two sulphur atoms gives it the name metabisulfite. The direct bond between the two sulphur atoms is referred to as the meta term. It is a well-known antioxidant that is utilised in medications. It is a solid powder that is white in hue.
, and its molecular weight is 190.107g/mol. It is an ionic compound of Sodium that contains Sulphur. Sodium metabisulfite is soluble in both water and glycerol. The positive charge of sodium contrasts with the negative charge of metabisulfite. Having two sulphur atoms gives it the name metabisulfite. The direct bond between the two sulphur atoms is referred to as the meta term. It is a well-known antioxidant that is utilised in medications. It is a solid powder that is white in hue.
- What is the full form of SMBS?
- Preparation Of SMBS
- Physical Properties Of SMBS
- Chemical properties of SMBS
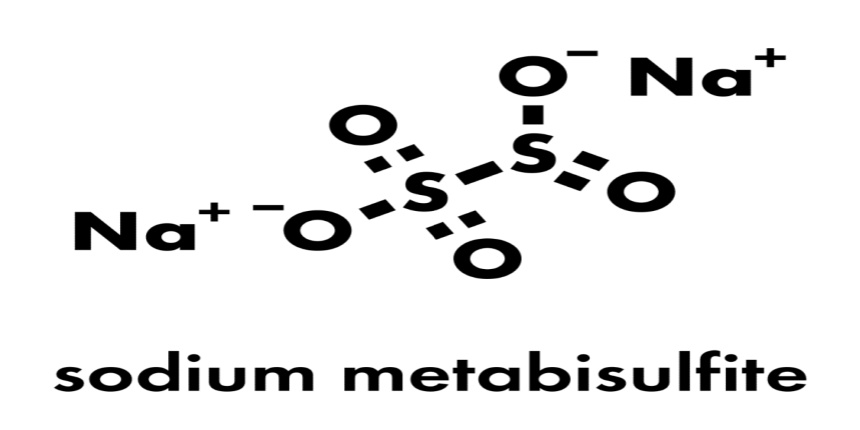
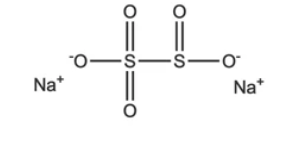
Preparation Of SMBS
Sulfur dioxide can be added to sodium hydroxide solution to create sodium metabisulfite. When tested in warm water, \[N{{a}_{2}}{{S}_{2}}{{O}_{5}}\] ![]() first forms a solid yellow precipitate. More \[S{{O}_{2}}\]
first forms a solid yellow precipitate. More \[S{{O}_{2}}\] ![]() causes the solid to dissolve, producing disulfite, which crystallises when cooled.
causes the solid to dissolve, producing disulfite, which crystallises when cooled.
\[S{{O}_{2}}~+\text{ }2\text{ }NaOH\text{ }\to \text{ }N{{a}_{2}}S{{O}_{3}}~+\text{ }{{H}_{2}}O\]
\[S{{O}_{2}}~+\text{ }N{{a}_{2}}S{{O}_{3}}~\to \text{ }N{{a}_{2}}{{S}_{2}}{{O}_{5}}\]
![]()
![]()
Physical Properties Of SMBS
In its solid state, sodium sulfite appears white or whitish-yellow.
It smells faintly unpleasant, rather like \[S{{O}_{2}}\]
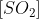 .
.\[N{{a}_{2}}{{S}_{2}}{{O}_{5}}\]
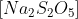 is a relatively soluble substance in water; its solubility equates to 65.3g/100mL at 20 degree Celsius.
is a relatively soluble substance in water; its solubility equates to 65.3g/100mL at 20 degree Celsius.While not very soluble in ethanol, this chemical is extremely soluble in glycerol.
Chemical properties of SMBS
Sodium metabisulfite releases sulphur dioxide gas, which has a solid and disagreeable odour when exposed to water. Humans may experience respiratory issues as a result of this gas.
This compound releases \[S{{O}_{2}}\]
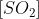 gas when exposed to potent acids like hydrochloric acid. This reaction's chemical equation is provided by:
gas when exposed to potent acids like hydrochloric acid. This reaction's chemical equation is provided by:
\[\mathbf{2HCl}\text{ }+\text{ }\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}{{\mathbf{S}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{5}}}~\to \text{ }\mathbf{2S}{{\mathbf{O}}_{\mathbf{2}}}~+\text{ }\mathbf{2NaCl}\text{ }+\text{ }{{\mathbf{H}}_{\mathbf{2}}}\mathbf{O}\]
![]()
When heated, sodium metabisulfite decomposes into sulphur dioxide and sodium sulfite. The reaction is as follows:
\[\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}{{\mathbf{S}}_{\mathbf{2}}}{{\mathbf{O}}_{\mathbf{5}}}~\to \text{ }\mathbf{S}{{\mathbf{O}}_{\mathbf{2}}}~+\text{ }\mathbf{N}{{\mathbf{a}}_{\mathbf{2}}}\mathbf{S}{{\mathbf{O}}_{\mathbf{3}}}\]
![]()